Nutrient regulation of the mTOR complex 1 signaling pathway
- PMID: 23694989
- PMCID: PMC3887879
- DOI: 10.1007/s10059-013-0138-2
Nutrient regulation of the mTOR complex 1 signaling pathway
Abstract
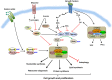
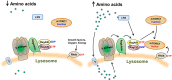
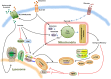
The mammalian target of rapamycin (mTOR) is an evolutionally conserved kinase which exists in two distinct structural and functional complexes, mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2). Of the two complexes, mTORC1 couples nutrient abundance to cell growth and proliferation by sensing and integrating a variety of inputs arising from amino acids, cellular stresses, energy status, and growth factors. Defects in mTORC1 regulation are implicated in the development of many metabolic diseases, including cancer and diabetes. Over the past decade, significant advances have been made in deciphering the complexity of the signaling processes contributing to mTORC1 regulation and function, but the mechanistic details are still not fully understood. In particular, how amino acid availability is sensed by cells and signals to mTORC1 remains unclear. In this review, we discuss the current understanding of nutrient-dependent control of mTORC1 signaling and will focus on the key components involved in amino acid signaling to mTORC1.
Figures
Similar articles
-
Nutrient signaling to mTOR and cell growth.Trends Biochem Sci. 2013 May;38(5):233-42. doi: 10.1016/j.tibs.2013.01.004. Epub 2013 Mar 1. Trends Biochem Sci. 2013. PMID: 23465396 Free PMC article. Review.
-
Spatial regulation of the mTORC1 system in amino acids sensing pathway.Acta Biochim Biophys Sin (Shanghai). 2011 Sep;43(9):671-9. doi: 10.1093/abbs/gmr066. Epub 2011 Jul 23. Acta Biochim Biophys Sin (Shanghai). 2011. PMID: 21785113 Free PMC article. Review.
-
Growing knowledge of the mTOR signaling network.Semin Cell Dev Biol. 2014 Dec;36:79-90. doi: 10.1016/j.semcdb.2014.09.011. Epub 2014 Sep 19. Semin Cell Dev Biol. 2014. PMID: 25242279 Free PMC article. Review.
-
mTOR-dependent cell survival mechanisms.Cold Spring Harb Perspect Biol. 2012 Dec 1;4(12):a008771. doi: 10.1101/cshperspect.a008771. Cold Spring Harb Perspect Biol. 2012. PMID: 23124837 Free PMC article. Review.
-
Mechanistic target of rapamycin: integrating growth factor and nutrient signaling in the collecting duct.Am J Physiol Renal Physiol. 2018 Sep 1;315(3):F413-F416. doi: 10.1152/ajprenal.00170.2018. Epub 2018 May 30. Am J Physiol Renal Physiol. 2018. PMID: 29846113 Review.
Cited by
-
Milk--A Nutrient System of Mammalian Evolution Promoting mTORC1-Dependent Translation.Int J Mol Sci. 2015 Jul 27;16(8):17048-87. doi: 10.3390/ijms160817048. Int J Mol Sci. 2015. PMID: 26225961 Free PMC article. Review.
-
Human ovarian tissue in-vitro culture: primordial follicle activation as a new strategy for female fertility preservation.Cytotechnology. 2022 Feb;74(1):1-15. doi: 10.1007/s10616-021-00510-2. Epub 2022 Jan 4. Cytotechnology. 2022. PMID: 35185282 Free PMC article. Review.
-
Essential Amino Acid Profile in Parenteral Nutrition Mixtures: Does It Meet Needs?Nutrients. 2018 Dec 6;10(12):1937. doi: 10.3390/nu10121937. Nutrients. 2018. PMID: 30563270 Free PMC article.
-
The catalytic subunit of the system L1 amino acid transporter (slc7a5) facilitates nutrient signalling in mouse skeletal muscle.PLoS One. 2014 Feb 26;9(2):e89547. doi: 10.1371/journal.pone.0089547. eCollection 2014. PLoS One. 2014. PMID: 24586861 Free PMC article.
-
Current models of mammalian target of rapamycin complex 1 (mTORC1) activation by growth factors and amino acids.Int J Mol Sci. 2014 Nov 13;15(11):20753-69. doi: 10.3390/ijms151120753. Int J Mol Sci. 2014. PMID: 25402640 Free PMC article. Review.
References
-
- Ashrafi K, Farazi TA, Gordon JI. (1998). A role for Saccharomyces cerevisiae fatty acid activation protein 4 in regulating protein N-myristoylation during entry into stationary phase. J Biol Chem. 273, 25864–25874 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

