Adipose tissue-derived multipotent stromal cells have a higher immunomodulatory capacity than their bone marrow-derived counterparts
- PMID: 23694810
- PMCID: PMC3673757
- DOI: 10.5966/sctm.2012-0184
Adipose tissue-derived multipotent stromal cells have a higher immunomodulatory capacity than their bone marrow-derived counterparts
Abstract
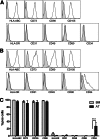
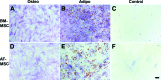
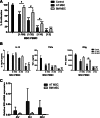
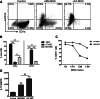
Adipose tissue-derived multipotent stromal cells (AT-MSCs) are studied as an alternative to bone marrow-derived multipotent stromal cells (BM-MSCs) for immunomodulatory treatment. In this study, we systematically compared the immunomodulatory capacities of BM-MSCs and AT-MSCs derived from age-matched donors. We found that BM-MSCs and AT-MSCs share a similar immunophenotype and capacity for in vitro multilineage differentiation. BM-MSCs and AT-MSCs showed comparable immunomodulatory effects as they were both able to suppress proliferation of stimulated peripheral blood mononuclear cells and to inhibit differentiation of monocyte-derived immature dendritic cells. However, at equal cell numbers, the AT-MSCs showed more potent immunomodulatory effects in both assays as compared with BM-MSCs. Moreover, AT-MSCs showed a higher level of secretion of cytokines that have been implicated in the immunomodulatory modes of action of multipotent stromal cells, such as interleukin-6 and transforming growth factor-β1. This is correlated with higher metabolic activity of AT-MSCs compared with BM-MSCs. We conclude that the immunomodulatory capacities of BM-MSCs and AT-MSCs are similar, but that differences in cytokine secretion cause AT-MSCs to have more potent immunomodulatory effects than BM-MSCs. Therefore, lower numbers of AT-MSCs evoke the same level of immunomodulation. These data indicate that AT-MSCs can be considered as a good alternative to BM-MSCs for immunomodulatory therapy.
Keywords: Adult human bone marrow; Bone marrow; Bone marrow stromal cells; Cellular therapy; Immunosuppression; Marrow stromal stem cells; Mesenchymal stem cells.
Figures
Similar articles
-
Comparative analysis of the immunomodulatory capacities of human bone marrow- and adipose tissue-derived mesenchymal stromal cells from the same donor.Cytotherapy. 2016 Oct;18(10):1297-311. doi: 10.1016/j.jcyt.2016.07.006. Cytotherapy. 2016. PMID: 27637760
-
Comparison of Antibacterial and Immunological Properties of Mesenchymal Stem/Stromal Cells from Equine Bone Marrow, Endometrium, and Adipose Tissue.Stem Cells Dev. 2018 Nov 1;27(21):1518-1525. doi: 10.1089/scd.2017.0241. Epub 2018 Sep 6. Stem Cells Dev. 2018. PMID: 30044182 Free PMC article.
-
Adipose tissue-derived mesenchymal stem cells are more potent suppressors of dendritic cells differentiation compared to bone marrow-derived mesenchymal stem cells.Immunol Lett. 2009 Sep 22;126(1-2):37-42. doi: 10.1016/j.imlet.2009.07.010. Epub 2009 Jul 30. Immunol Lett. 2009. PMID: 19647021
-
Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells.Stem Cells Dev. 2012 Sep 20;21(14):2724-52. doi: 10.1089/scd.2011.0722. Epub 2012 May 9. Stem Cells Dev. 2012. PMID: 22468918 Review.
-
Immunomodulatory properties of human adult and fetal multipotent mesenchymal stem cells.J Biomed Sci. 2011 Jul 18;18(1):49. doi: 10.1186/1423-0127-18-49. J Biomed Sci. 2011. PMID: 21762539 Free PMC article. Review.
Cited by
-
Preclinical evaluation of the immunomodulatory properties of cardiac adipose tissue progenitor cells using umbilical cord blood mesenchymal stem cells: a direct comparative study.Biomed Res Int. 2015;2015:439808. doi: 10.1155/2015/439808. Epub 2015 Mar 10. Biomed Res Int. 2015. PMID: 25861626 Free PMC article.
-
Stem Cells for Cutaneous Wound Healing.Biomed Res Int. 2015;2015:285869. doi: 10.1155/2015/285869. Epub 2015 Jun 2. Biomed Res Int. 2015. PMID: 26137471 Free PMC article. Review.
-
Inhibiting DNA methylation as a strategy to enhance adipose-derived stem cells differentiation: Focus on the role of Akt/mTOR and Wnt/β-catenin pathways on adipogenesis.Front Cell Dev Biol. 2022 Sep 2;10:926180. doi: 10.3389/fcell.2022.926180. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36120582 Free PMC article.
-
Immunoregulation by mesenchymal stem cells: biological aspects and clinical applications.J Immunol Res. 2015;2015:394917. doi: 10.1155/2015/394917. Epub 2015 Apr 19. J Immunol Res. 2015. PMID: 25961059 Free PMC article. Review.
-
Application of adipose-derived stem cells in ischemic heart disease: theory, potency, and advantage.Front Cardiovasc Med. 2024 Jan 19;11:1324447. doi: 10.3389/fcvm.2024.1324447. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 38312236 Free PMC article. Review.
References
-
- Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells: The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. - PubMed
-
- Di Nicola M, Carlo-Stella C, Magni M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–3843. - PubMed
-
- Marigo I, Dazzi F. The immunomodulatory properties of mesenchymal stem cells. Semin Immunopathol. 2011;33:593–602. - PubMed
-
- Nauta AJ, Kruisselbrink AB, Lurvink E, et al. Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. J Immunol. 2006;177:2080–2087. - PubMed
-
- Djouad F, Charbonnier LM, Bouffi C, et al. Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem Cells. 2007;25:2025–2032. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

