Clearance of Pseudomonas aeruginosa foreign-body biofilm infections through reduction of the cyclic Di-GMP level in the bacteria
- PMID: 23690403
- PMCID: PMC3719571
- DOI: 10.1128/IAI.00332-13
Clearance of Pseudomonas aeruginosa foreign-body biofilm infections through reduction of the cyclic Di-GMP level in the bacteria
Abstract
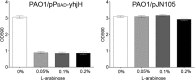
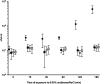
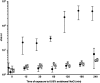
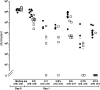
Opportunistic pathogenic bacteria can engage in biofilm-based infections that evade immune responses and develop into chronic conditions. Because conventional antimicrobials cannot efficiently eradicate biofilms, there is an urgent need to develop alternative measures to combat biofilm infections. It has recently been established that the secondary messenger cyclic diguanosine monophosphate (c-di-GMP) functions as a positive regulator of biofilm formation in several different bacteria. In the present study we investigated whether manipulation of the c-di-GMP level in bacteria potentially can be used for biofilm control in vivo. We constructed a Pseudomonas aeruginosa strain in which a reduction in the c-di-GMP level can be achieved via induction of the Escherichia coli YhjH c-di-GMP phosphodiesterase. Initial experiments showed that induction of yhjH expression led to dispersal of the majority of the bacteria in in vitro-grown P. aeruginosa biofilms. Subsequently, we demonstrated that P. aeruginosa biofilms growing on silicone implants, located in the peritoneal cavity of mice, dispersed after induction of the YhjH protein. Bacteria accumulated temporarily in the spleen after induction of biofilm dispersal, but the mice tolerated the dispersed bacteria well. The present work provides proof of the concept that modulation of the c-di-GMP level in bacteria is a viable strategy for biofilm control.
Figures




Similar articles
-
Induction of Native c-di-GMP Phosphodiesterases Leads to Dispersal of Pseudomonas aeruginosa Biofilms.Antimicrob Agents Chemother. 2021 Mar 18;65(4):e02431-20. doi: 10.1128/AAC.02431-20. Print 2021 Mar 18. Antimicrob Agents Chemother. 2021. PMID: 33495218 Free PMC article.
-
In vitro and in vivo generation and characterization of Pseudomonas aeruginosa biofilm-dispersed cells via c-di-GMP manipulation.Nat Protoc. 2015 Aug;10(8):1165-80. doi: 10.1038/nprot.2015.067. Epub 2015 Jul 9. Nat Protoc. 2015. PMID: 26158442
-
Heterogeneity in surface sensing suggests a division of labor in Pseudomonas aeruginosa populations.Elife. 2019 Jun 10;8:e45084. doi: 10.7554/eLife.45084. Elife. 2019. PMID: 31180327 Free PMC article.
-
Biofilms and Cyclic di-GMP (c-di-GMP) Signaling: Lessons from Pseudomonas aeruginosa and Other Bacteria.J Biol Chem. 2016 Jun 10;291(24):12547-12555. doi: 10.1074/jbc.R115.711507. Epub 2016 Apr 21. J Biol Chem. 2016. PMID: 27129226 Free PMC article. Review.
-
c-di-GMP and its Effects on Biofilm Formation and Dispersion: a Pseudomonas Aeruginosa Review.Microbiol Spectr. 2015 Apr;3(2):MB-0003-2014. doi: 10.1128/microbiolspec.MB-0003-2014. Microbiol Spectr. 2015. PMID: 26104694 Free PMC article. Review.
Cited by
-
Biofilm tolerance, resistance and infections increasing threat of public health.Microb Cell. 2023 Sep 26;10(11):233-247. doi: 10.15698/mic2023.11.807. eCollection 2023 Nov 6. Microb Cell. 2023. PMID: 37933277 Free PMC article. Review.
-
Identification of a diguanylate cyclase and its role in Porphyromonas gingivalis virulence.Infect Immun. 2014 Jul;82(7):2728-35. doi: 10.1128/IAI.00084-14. Epub 2014 Apr 14. Infect Immun. 2014. PMID: 24733094 Free PMC article.
-
BdlA, DipA and induced dispersion contribute to acute virulence and chronic persistence of Pseudomonas aeruginosa.PLoS Pathog. 2014 Jun 5;10(6):e1004168. doi: 10.1371/journal.ppat.1004168. eCollection 2014 Jun. PLoS Pathog. 2014. PMID: 24901523 Free PMC article.
-
Targeting Biofilms Therapy: Current Research Strategies and Development Hurdles.Microorganisms. 2020 Aug 11;8(8):1222. doi: 10.3390/microorganisms8081222. Microorganisms. 2020. PMID: 32796745 Free PMC article. Review.
-
Identification of small molecules that interfere with c-di-GMP signaling and induce dispersal of Pseudomonas aeruginosa biofilms.NPJ Biofilms Microbiomes. 2021 Jul 9;7(1):59. doi: 10.1038/s41522-021-00225-4. NPJ Biofilms Microbiomes. 2021. PMID: 34244523 Free PMC article.
References
-
- Costerton JW, Stewart PS, Greenberg EP. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322 - PubMed
-
- Hentzer M, Wu H, Andersen JB, Riedel K, Rasmussen TB, Bagge N, Kumar N, Schembri MA, Song Z, Kristoffersen P, Manefield M, Costerton JW, Molin S, Eberl L, Steinberg P, Kjelleberg S, Hoiby N, Givskov M. 2003. Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J. 22:3803–3815 - PMC - PubMed
-
- Bjarnsholt T, Jensen PO, Burmolle M, Hentzer M, Haagensen JA, Hougen HP, Calum H, Madsen KG, Moser C, Molin S, Hoiby N, Givskov M. 2005. Pseudomonas aeruginosa tolerance to tobramycin, hydrogen peroxide and polymorphonuclear leukocytes is quorum-sensing dependent. Microbiology 151:373–383 - PubMed
-
- Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. 1995. Microbial biofilms. Annu. Rev. Microbiol. 49:711–745 - PubMed
-
- Koch C, Hoiby N. 1993. Pathogenesis of cystic fibrosis. Lancet 341:1065–1069 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

