Thrombin, a mediator of cerebrovascular inflammation in AD and hypoxia
- PMID: 23675346
- PMCID: PMC3648692
- DOI: 10.3389/fnagi.2013.00019
Thrombin, a mediator of cerebrovascular inflammation in AD and hypoxia
Abstract
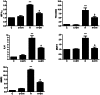
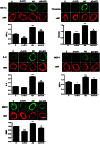
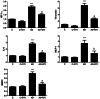
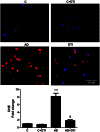
Considerable evidence implicates hypoxia and vascular inflammation in Alzheimer's disease (AD). Thrombin, a multifunctional inflammatory mediator, is demonstrable in the brains of AD patients both in the vessel walls and senile plaques. Hypoxia-inducible factor 1α (HIF-1α), a key regulator of the cellular response to hypoxia, is also upregulated in the vasculature of human AD brains. The objective of this study is to investigate inflammatory protein expression in the cerebrovasculature of transgenic AD mice and to explore the role of thrombin as a mediator of cerebrovascular inflammation and oxidative stress in AD and in hypoxia-induced changes in brain endothelial cells. Immunofluorescent analysis of the cerebrovasculature in AD mice demonstrates significant (p < 0.01-0.001) increases in thrombin, HIF-1α, interleukin-6 (IL-6), monocyte chemoattractant protein-1 (MCP-1), matrix metalloproteinases (MMPs), and reactive oxygen species (ROS) compared to controls. Administration of the thrombin inhibitor dabigatran (100 mg/kg) to AD mice for 34 weeks significantly decreases expression of inflammatory proteins and ROS. Exposure of cultured brain endothelial cells to hypoxia for 6 h causes an upregulation of thrombin, HIF-1α, MCP-1, IL-6, and MMP2 and ROS. Treatment of endothelial cells with the dabigatran (1 nM) reduces ROS generation and inflammatory protein expression (p < 0.01-0.001). The data demonstrate that inhibition of thrombin in culture blocks the increase in inflammatory protein expression and ROS generation evoked by hypoxia. Also, administration of dabigatran to transgenic AD mice diminishes ROS levels in brain and reduces cerebrovascular expression of inflammatory proteins. Taken together, these results suggest that inhibiting thrombin generation could have therapeutic value in AD and other disorders where hypoxia, inflammation, and oxidative stress are involved.
Keywords: Alzheimer's disease; dabigatran; endothelial cells; hypoxia; neuroinflammation; thrombin.
Figures
Similar articles
-
Thrombin Signaling Contributes to High Glucose-Induced Injury of Human Brain Microvascular Endothelial Cells.J Alzheimers Dis. 2021;79(1):211-224. doi: 10.3233/JAD-200658. J Alzheimers Dis. 2021. PMID: 33252072
-
p38 MAPK: a mediator of hypoxia-induced cerebrovascular inflammation.J Alzheimers Dis. 2012;32(3):587-97. doi: 10.3233/JAD-2012-120829. J Alzheimers Dis. 2012. PMID: 22886009
-
Brain microvasculature and hypoxia-related proteins in Alzheimer's disease.Int J Clin Exp Pathol. 2011 Aug 15;4(6):616-27. Epub 2011 Jun 18. Int J Clin Exp Pathol. 2011. PMID: 21904637 Free PMC article.
-
Targeting thrombin: an inflammatory neurotoxin in Alzheimer's disease.J Alzheimers Dis. 2014;42 Suppl 4:S537-44. doi: 10.3233/JAD-141557. J Alzheimers Dis. 2014. PMID: 25079808 Review.
-
Thrombin, a Mediator of Coagulation, Inflammation, and Neurotoxicity at the Neurovascular Interface: Implications for Alzheimer's Disease.Front Neurosci. 2020 Jul 24;14:762. doi: 10.3389/fnins.2020.00762. eCollection 2020. Front Neurosci. 2020. PMID: 32792902 Free PMC article. Review.
Cited by
-
Key brain cell interactions and contributions to the pathogenesis of Alzheimer's disease.Front Cell Dev Biol. 2022 Nov 29;10:1036123. doi: 10.3389/fcell.2022.1036123. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36523504 Free PMC article. Review.
-
High ferritin levels have major effects on the morphology of erythrocytes in Alzheimer's disease.Front Aging Neurosci. 2013 Dec 6;5:88. doi: 10.3389/fnagi.2013.00088. eCollection 2013. Front Aging Neurosci. 2013. PMID: 24367334 Free PMC article.
-
Long-Term Dabigatran Treatment Delays Alzheimer's Disease Pathogenesis in the TgCRND8 Mouse Model.J Am Coll Cardiol. 2019 Oct 15;74(15):1910-1923. doi: 10.1016/j.jacc.2019.07.081. J Am Coll Cardiol. 2019. PMID: 31601371 Free PMC article.
-
Thrombin, a Key Driver of Pathological Inflammation in the Brain.Cells. 2023 Apr 23;12(9):1222. doi: 10.3390/cells12091222. Cells. 2023. PMID: 37174621 Free PMC article. Review.
-
Dabigatran reduces thrombin-induced neuroinflammation and AD markers in vitro: Therapeutic relevance for Alzheimer's disease.Cereb Circ Cogn Behav. 2021 May 6;2:100014. doi: 10.1016/j.cccb.2021.100014. eCollection 2021. Cereb Circ Cogn Behav. 2021. PMID: 36324711 Free PMC article.
References
-
- Akiyama H., Ikeda K., Kondo H., McGeer P. L. (1992). Thrombin accumulation in brains of patients with Alzheimer's disease. Neurosci. Lett. 146, 152–154 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

