LGP2 plays a critical role in sensitizing mda-5 to activation by double-stranded RNA
- PMID: 23671710
- PMCID: PMC3650065
- DOI: 10.1371/journal.pone.0064202
LGP2 plays a critical role in sensitizing mda-5 to activation by double-stranded RNA
Abstract
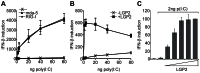
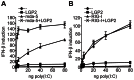
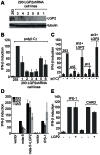
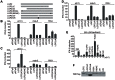
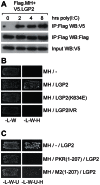
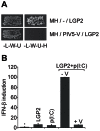
The DExD/H box RNA helicases retinoic acid-inducible gene-I (RIG-I) and melanoma differentiation associated gene-5 (mda-5) sense viral RNA in the cytoplasm of infected cells and activate signal transduction pathways that trigger the production of type I interferons (IFNs). Laboratory of genetics and physiology 2 (LGP2) is thought to influence IFN production by regulating the activity of RIG-I and mda-5, although its mechanism of action is not known and its function is controversial. Here we show that expression of LGP2 potentiates IFN induction by polyinosinic-polycytidylic acid [poly(I:C)], commonly used as a synthetic mimic of viral dsRNA, and that this is particularly significant at limited levels of the inducer. The observed enhancement is mediated through co-operation with mda-5, which depends upon LGP2 for maximal activation in response to poly(I:C). This co-operation is dependent upon dsRNA binding by LGP2, and the presence of helicase domain IV, both of which are required for LGP2 to interact with mda-5. In contrast, although RIG-I can also be activated by poly(I:C), LGP2 does not have the ability to enhance IFN induction by RIG-I, and instead acts as an inhibitor of RIG-I-dependent poly(I:C) signaling. Thus the level of LGP2 expression is a critical factor in determining the cellular sensitivity to induction by dsRNA, and this may be important for rapid activation of the IFN response at early times post-infection when the levels of inducer are low.
Conflict of interest statement
Figures
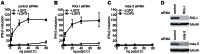
Similar articles
-
The RIG-I-like receptor LGP2 inhibits Dicer-dependent processing of long double-stranded RNA and blocks RNA interference in mammalian cells.EMBO J. 2018 Feb 15;37(4):e97479. doi: 10.15252/embj.201797479. Epub 2018 Jan 19. EMBO J. 2018. PMID: 29351913 Free PMC article.
-
Paramyxovirus V proteins interact with the RNA Helicase LGP2 to inhibit RIG-I-dependent interferon induction.J Virol. 2012 Apr;86(7):3411-21. doi: 10.1128/JVI.06405-11. Epub 2012 Feb 1. J Virol. 2012. PMID: 22301134 Free PMC article.
-
The RIG-I-like receptor LGP2 recognizes the termini of double-stranded RNA.J Biol Chem. 2009 May 15;284(20):13881-13891. doi: 10.1074/jbc.M900818200. Epub 2009 Mar 11. J Biol Chem. 2009. PMID: 19278996 Free PMC article.
-
Retinoic acid-inducible gene-I-like receptors.J Interferon Cytokine Res. 2011 Jan;31(1):27-31. doi: 10.1089/jir.2010.0057. Epub 2010 Oct 15. J Interferon Cytokine Res. 2011. PMID: 20950133 Review.
-
Proofreading mechanisms of the innate immune receptor RIG-I: distinguishing self and viral RNA.Biochem Soc Trans. 2024 Jun 26;52(3):1131-1148. doi: 10.1042/BST20230724. Biochem Soc Trans. 2024. PMID: 38884803 Free PMC article. Review.
Cited by
-
USP26 suppresses type I interferon signaling by targeting TRAF3 for deubiquitination.PLoS One. 2024 Jul 26;19(7):e0307776. doi: 10.1371/journal.pone.0307776. eCollection 2024. PLoS One. 2024. PMID: 39058724 Free PMC article.
-
Influenza virus activation of the interferon system.Virus Res. 2015 Nov 2;209:11-22. doi: 10.1016/j.virusres.2015.02.003. Epub 2015 Feb 9. Virus Res. 2015. PMID: 25678267 Free PMC article. Review.
-
Amplification of immunity by engineering chicken MDA5 combined with the C terminal domain (CTD) of RIG-I.Appl Microbiol Biotechnol. 2022 Feb;106(4):1599-1613. doi: 10.1007/s00253-022-11806-4. Epub 2022 Feb 7. Appl Microbiol Biotechnol. 2022. PMID: 35129655
-
Mechanisms of Non-segmented Negative Sense RNA Viral Antagonism of Host RIG-I-Like Receptors.J Mol Biol. 2019 Oct 4;431(21):4281-4289. doi: 10.1016/j.jmb.2019.06.002. Epub 2019 Jun 14. J Mol Biol. 2019. PMID: 31202887 Free PMC article. Review.
-
Comparative analysis of viral RNA signatures on different RIG-I-like receptors.Elife. 2016 Mar 24;5:e11275. doi: 10.7554/eLife.11275. Elife. 2016. PMID: 27011352 Free PMC article.
References
-
- Kumagai Y, Akira S (2010) Identification and functions of pattern-recognition receptors. J Allergy Clin Immunol. 125: 985–992. - PubMed
-
- Hornung V, Ellegast J, Kim S, Brzozka K, Jung A, et al. (2006) 5′-Triphosphate RNA is the ligand for RIG-I. Science 314: 994–997. - PubMed
-
- Pichlmair A, Schulz O, Tan CP, Naslund TI, Liljestrom P, et al. (2006) RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science 314: 997–1001. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

