Infusion of imaging and therapeutic molecules into the plant virus-based carrier cowpea mosaic virus: cargo-loading and delivery
- PMID: 23665254
- PMCID: PMC3815978
- DOI: 10.1016/j.jconrel.2013.04.023
Infusion of imaging and therapeutic molecules into the plant virus-based carrier cowpea mosaic virus: cargo-loading and delivery
Abstract
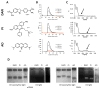
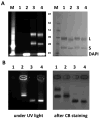
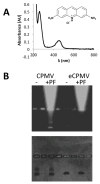
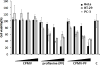
This work is focused on the development of a plant virus-based carrier system for cargo delivery, specifically 30nm-sized cowpea mosaic virus (CPMV). Whereas previous reports described the engineering of CPMV through genetic or chemical modification, we report a non-covalent infusion technique that facilitates efficient cargo loading. Infusion and retention of 130-155 fluorescent dye molecules per CPMV using DAPI (4',6-diamidino-2-phenylindole dihydrochloride), propidium iodide (3,8-diamino-5-[3-(diethylmethylammonio)propyl]-6-phenylphenanthridinium diiodide), and acridine orange (3,6-bis(dimethylamino)acridinium chloride), as well as 140 copies of therapeutic payload proflavine (PF, acridine-3,6-diamine hydrochloride), is reported. Loading is achieved through interaction of the cargo with the CPMV's encapsidated RNA molecules. The loading mechanism is specific; empty RNA-free eCPMV nanoparticles could not be loaded. Cargo-infused CPMV nanoparticles remain chemically active, and surface lysine residues were covalent modified with dyes leading to the development of dual-functional CPMV carrier systems. We demonstrate cargo-delivery to a panel of cancer cells (cervical, breast, and colon): CPMV nanoparticles enter cells via the surface marker vimentin, the nanoparticles target the endolysosome, where the carrier is degraded and the cargo is released allowing imaging and/or cell killing. In conclusion, we demonstrate cargo-infusion and delivery to cells; the methods discussed provide a useful means for functionalization of CPMV toward its application as drug and/or contrast agent delivery vehicle.
Keywords: Cowpea mosaic virus; Drug delivery; Infusion; Viral nanoparticle.
Copyright © 2013 Elsevier B.V. All rights reserved.
Figures


Similar articles
-
Cowpea mosaic virus stimulates antitumor immunity through recognition by multiple MYD88-dependent toll-like receptors.Biomaterials. 2021 Aug;275:120914. doi: 10.1016/j.biomaterials.2021.120914. Epub 2021 May 25. Biomaterials. 2021. PMID: 34126409 Free PMC article.
-
Cowpea Mosaic Virus Nanoparticles and Empty Virus-Like Particles Show Distinct but Overlapping Immunostimulatory Properties.J Virol. 2019 Oct 15;93(21):e00129-19. doi: 10.1128/JVI.00129-19. Print 2019 Nov 1. J Virol. 2019. PMID: 31375592 Free PMC article.
-
CPMV-DOX delivers.Mol Pharm. 2013 Jan 7;10(1):3-10. doi: 10.1021/mp3002057. Epub 2012 Aug 6. Mol Pharm. 2013. PMID: 22827473 Free PMC article.
-
Cowpea mosaic virus nanoparticles for cancer imaging and therapy.Adv Drug Deliv Rev. 2019 May;145:130-144. doi: 10.1016/j.addr.2019.04.005. Epub 2019 Apr 17. Adv Drug Deliv Rev. 2019. PMID: 31004625 Review.
-
Hybrid assembly of CPMV viruses and surface characteristics of different mutants.Curr Top Microbiol Immunol. 2009;327:59-69. doi: 10.1007/978-3-540-69379-6_3. Curr Top Microbiol Immunol. 2009. PMID: 19198570 Review.
Cited by
-
Dawn of advanced molecular medicine: nanotechnological advancements in cancer imaging and therapy.Crit Rev Oncog. 2014;19(3-4):143-76. doi: 10.1615/critrevoncog.2014011601. Crit Rev Oncog. 2014. PMID: 25271430 Free PMC article. Review.
-
Virus-Based Nanoparticles as Versatile Nanomachines.Annu Rev Virol. 2015 Nov;2(1):379-401. doi: 10.1146/annurev-virology-100114-055141. Epub 2015 Sep 25. Annu Rev Virol. 2015. PMID: 26958921 Free PMC article. Review.
-
In situ vaccination with cowpea mosaic virus nanoparticles suppresses metastatic cancer.Nat Nanotechnol. 2016 Mar;11(3):295-303. doi: 10.1038/nnano.2015.292. Epub 2015 Dec 21. Nat Nanotechnol. 2016. PMID: 26689376 Free PMC article.
-
Enhancing and initiating phage-based therapies.Bacteriophage. 2014 Dec 15;4(4):e961869. doi: 10.4161/21597073.2014.961869. eCollection 2014. Bacteriophage. 2014. PMID: 26713220 Free PMC article.
-
Biomedical and Catalytic Opportunities of Virus-Like Particles in Nanotechnology.Adv Virus Res. 2017;97:1-60. doi: 10.1016/bs.aivir.2016.09.002. Epub 2016 Nov 8. Adv Virus Res. 2017. PMID: 28057256 Free PMC article. Review.
References
-
- Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J Control Release. 2000;65(1–2):271–284. - PubMed
-
- Perrault SD, Walkey C, Jennings T, Fischer HC, Chan WC. Mediating tumor targeting efficiency of nanoparticles through design. Nano letters. 2009;9(5):1909–1915. - PubMed
-
- Ruoslahti E. Vascular zip codes in angiogenesis and metastasis. Biochem Soc Trans. 2004;32(Pt3):397–402. - PubMed
-
- Chitale R. Merck hopes to extend gardasil vaccine to men. J Natl Cancer Inst. 2009;101(4):222–223. - PubMed
-
- Liu TC, Galanis E, Kirn D. Clinical trial results with oncolytic virotherapy: A century of promise, a decade of progress. Nat Clin Pract Oncol. 2007;4(2):101–117. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

