Platelet-derived growth factor alpha and beta receptors have overlapping functional activities towards fibroblasts
- PMID: 23663505
- PMCID: PMC3667071
- DOI: 10.1186/1755-1536-6-10
Platelet-derived growth factor alpha and beta receptors have overlapping functional activities towards fibroblasts
Abstract
Background: Platelet-derived growth factor (PDGF) signalling is essential for many key cellular processes in mesenchymal cells. As there is redundancy in signalling between the five PDGF ligand isoforms and three PDGF receptor isoforms, and deletion of either of the receptors in vivo produces an embryonic lethal phenotype, it is not know which ligand and receptor combinations mediate specific cellular functions. Fibroblasts are key mediators in wound healing and tissues repair. Recent clinical trials using broad spectrum tyrosine kinase inhibitors in fibrotic diseases have highlighted the need to further examine the specific cellular roles each of the tyrosine kinases plays in fibrotic processes. In this study, we used PDGFR-specific neutralising antibodies to dissect out receptor-specific signalling events in fibroblasts in vitro, to further understand key cellular processes involved in wound healing and tissue repair.
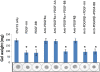
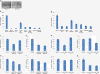
Results: Neutralising antibodies against PDGFRs were shown to block signalling through PDGFRα and PDGFRβ receptors, reduce human PDGF-AA and PDGF-BB-induced collagen gel remodelling in dermal fibroblasts, and reduce migration stimulated by all PDGF ligands in human dermal and lung fibroblasts.
Conclusions: PDGFRα and PDGFRβ neutralising antibodies can be a useful tool in studying PDGFR isoform-specific cellular events.
Figures



Similar articles
-
The role of small molecule platelet-derived growth factor receptor (PDGFR) inhibitors in the treatment of neoplastic disorders.Pharmacol Res. 2018 Mar;129:65-83. doi: 10.1016/j.phrs.2018.01.021. Epub 2018 Feb 3. Pharmacol Res. 2018. PMID: 29408302 Review.
-
Both platelet-derived growth factor receptor (PDGFR)-alpha and PDGFR-beta promote murine fibroblast cell migration.Biochem Biophys Res Commun. 2001 Apr 6;282(3):697-700. doi: 10.1006/bbrc.2001.4622. Biochem Biophys Res Commun. 2001. PMID: 11401517
-
Deletion of the PDGFR-beta gene affects key fibroblast functions important for wound healing.J Biol Chem. 2005 Mar 11;280(10):9375-89. doi: 10.1074/jbc.M413081200. Epub 2004 Dec 6. J Biol Chem. 2005. PMID: 15590688
-
Roles of PDGF/PDGFR signaling in various organs.Korean J Physiol Pharmacol. 2024 Oct 31. doi: 10.4196/kjpp.24.309. Online ahead of print. Korean J Physiol Pharmacol. 2024. PMID: 39482238
-
Oncogenic derivatives of platelet-derived growth factor receptors.Cell Mol Life Sci. 2004 Dec;61(23):2912-23. doi: 10.1007/s00018-004-4272-z. Cell Mol Life Sci. 2004. PMID: 15583853 Review.
Cited by
-
Single-cell analyses reveal early thymic progenitors and pre-B cells in zebrafish.J Exp Med. 2022 Sep 5;219(9):e20220038. doi: 10.1084/jem.20220038. Epub 2022 Aug 8. J Exp Med. 2022. PMID: 35938989 Free PMC article.
-
PDGFRα Is a Key Regulator of T1 and T3's Differential Effect on SMA Expression in Human Corneal Fibroblasts.Invest Ophthalmol Vis Sci. 2017 Feb 1;58(2):1179-1186. doi: 10.1167/iovs.16-20016. Invest Ophthalmol Vis Sci. 2017. PMID: 28245298 Free PMC article.
-
Crosstalk with lung fibroblasts shapes the growth and therapeutic response of mesothelioma cells.Cell Death Dis. 2023 Nov 8;14(11):725. doi: 10.1038/s41419-023-06240-x. Cell Death Dis. 2023. PMID: 37938546 Free PMC article.
-
Contraction Measurements Using Three-Dimensional Fibrillar Collagen Gel Lattices.Methods Mol Biol. 2021;2299:109-114. doi: 10.1007/978-1-0716-1382-5_7. Methods Mol Biol. 2021. PMID: 34028737
-
Unraveling SSc Pathophysiology; The Myofibroblast.Front Immunol. 2018 Nov 13;9:2452. doi: 10.3389/fimmu.2018.02452. eCollection 2018. Front Immunol. 2018. PMID: 30483246 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

