Prominent role for T cell-derived tumour necrosis factor for sustained control of Mycobacterium tuberculosis infection
- PMID: 23657146
- PMCID: PMC3648802
- DOI: 10.1038/srep01809
Prominent role for T cell-derived tumour necrosis factor for sustained control of Mycobacterium tuberculosis infection
Abstract
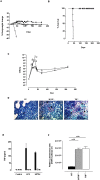
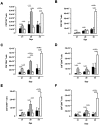
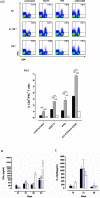
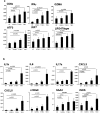
Tumour Necrosis Factor (TNF) is critical for host control of M. tuberculosis, but the relative contribution of TNF from innate and adaptive immune responses during tuberculosis infection is unclear. Myeloid versus T-cell-derived TNF function in tuberculosis was investigated using cell type-specific TNF deletion. Mice deficient for TNF expression in macrophages/neutrophils displayed early, transient susceptibility to M. tuberculosis but recruited activated, TNF-producing CD4(+) and CD8(+) T-cells and controlled chronic infection. Strikingly, deficient TNF expression in T-cells resulted in early control but susceptibility and eventual mortality during chronic infection with increased pulmonary pathology. TNF inactivation in both myeloid and T-cells rendered mice critically susceptible to infection with a phenotype resembling complete TNF deficient mice, indicating that myeloid and T-cells are the primary TNF sources collaborating for host control of tuberculosis. Thus, while TNF from myeloid cells mediates early immune function, T-cell derived TNF is essential to sustain protection during chronic tuberculosis infection.
Figures


Similar articles
-
Protective CD4 T cells targeting cryptic epitopes of Mycobacterium tuberculosis resist infection-driven terminal differentiation.J Immunol. 2014 Apr 1;192(7):3247-58. doi: 10.4049/jimmunol.1300283. Epub 2014 Feb 26. J Immunol. 2014. PMID: 24574499
-
Role of Granulocyte-Macrophage Colony-Stimulating Factor Production by T Cells during Mycobacterium tuberculosis Infection.mBio. 2017 Oct 24;8(5):e01514-17. doi: 10.1128/mBio.01514-17. mBio. 2017. PMID: 29066547 Free PMC article.
-
Critical and independent role for SOCS3 in either myeloid or T cells in resistance to Mycobacterium tuberculosis.PLoS Pathog. 2013;9(7):e1003442. doi: 10.1371/journal.ppat.1003442. Epub 2013 Jul 4. PLoS Pathog. 2013. PMID: 23853585 Free PMC article.
-
Myeloid derived hypoxia inducible factor 1-alpha is required for protection against pulmonary Aspergillus fumigatus infection.PLoS Pathog. 2014 Sep 25;10(9):e1004378. doi: 10.1371/journal.ppat.1004378. eCollection 2014 Sep. PLoS Pathog. 2014. PMID: 25255025 Free PMC article.
-
Neutrophils in tuberculosis: friend or foe?Trends Immunol. 2012 Jan;33(1):14-25. doi: 10.1016/j.it.2011.10.003. Epub 2011 Nov 15. Trends Immunol. 2012. PMID: 22094048 Review.
Cited by
-
T cell-derived tumor necrosis factor induces cytotoxicity by activating RIPK1-dependent target cell death.JCI Insight. 2021 Dec 22;6(24):e148643. doi: 10.1172/jci.insight.148643. JCI Insight. 2021. PMID: 34752416 Free PMC article.
-
A New Venue of TNF Targeting.Int J Mol Sci. 2018 May 11;19(5):1442. doi: 10.3390/ijms19051442. Int J Mol Sci. 2018. PMID: 29751683 Free PMC article. Review.
-
Mycobacterial Dormancy Systems and Host Responses in Tuberculosis.Front Immunol. 2017 Feb 15;8:84. doi: 10.3389/fimmu.2017.00084. eCollection 2017. Front Immunol. 2017. PMID: 28261197 Free PMC article. Review.
-
Identification of a TNF-α inducer MIC3 originating from the microneme of non-cystogenic, virulent Toxoplasma gondii.Sci Rep. 2016 Dec 21;6:39407. doi: 10.1038/srep39407. Sci Rep. 2016. PMID: 28000706 Free PMC article.
-
IL-21 signaling is essential for optimal host resistance against Mycobacterium tuberculosis infection.Sci Rep. 2016 Nov 7;6:36720. doi: 10.1038/srep36720. Sci Rep. 2016. PMID: 27819295 Free PMC article.
References
-
- Roach T. I., Barton C. H., Chatterjee D. & Blackwell J. M. Macrophage activation: lipoarabinomannan from avirulent and virulent strains of Mycobacterium tuberculosis differentially induces the early genes c-fos, KC, JE, and tumor necrosis factor-alpha. J Immunol 150, 1886–1896 (1993). - PubMed
-
- Falcone V., Bassey E. B., Toniolo A., Conaldi P. G. & Collins F. M. Differential release of tumor necrosis factor-alpha from murine peritoneal macrophages stimulated with virulent and avirulent species of mycobacteria. FEMS Immunol Med Microbiol 8, 225–232 (1994). - PubMed
-
- Oswald I. P., Dozois C. M., Fournout S., Petit J. F. & Lemaire G. Tumor necrosis factor is required for the priming of peritoneal macrophages by trehalose dimycolate. Eur Cytokine Netw 10, 533–540 (1999). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

