Chemotherapy-induced bone marrow nerve injury impairs hematopoietic regeneration
- PMID: 23644514
- PMCID: PMC3964478
- DOI: 10.1038/nm.3155
Chemotherapy-induced bone marrow nerve injury impairs hematopoietic regeneration
Abstract
Anticancer chemotherapy drugs challenge hematopoietic tissues to regenerate but commonly produce long-term sequelae. Chemotherapy-induced deficits in hematopoietic stem or stromal cell function have been described, but the mechanisms mediating hematopoietic dysfunction remain unclear. Administration of multiple cycles of cisplatin chemotherapy causes substantial sensory neuropathy. Here we demonstrate that chemotherapy-induced nerve injury in the bone marrow of mice is a crucial lesion impairing hematopoietic regeneration. Using pharmacological and genetic models, we show that the selective loss of adrenergic innervation in the bone marrow alters its regeneration after genotoxic insult. Sympathetic nerves in the marrow promote the survival of constituents of the stem cell niche that initiate recovery. Neuroprotection by deletion of Trp53 in sympathetic neurons or neuroregeneration by administration of 4-methylcatechol or glial-derived neurotrophic factor (GDNF) promotes hematopoietic recovery. These results demonstrate the potential benefit of adrenergic nerve protection for shielding hematopoietic niches from injury.
Conflict of interest statement
The authors declare no competing financial interests. Correspondence and requests for materials should be addressed to P.S.F. (
Figures

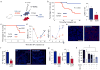
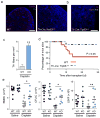
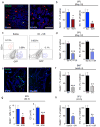
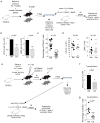

Comment in
-
It takes nerves to recover from chemotherapy.Nat Med. 2013 Jun;19(6):669-71. doi: 10.1038/nm.3231. Nat Med. 2013. PMID: 23744143 No abstract available.
Similar articles
-
It takes nerves to recover from chemotherapy.Nat Med. 2013 Jun;19(6):669-71. doi: 10.1038/nm.3231. Nat Med. 2013. PMID: 23744143 No abstract available.
-
Sympathetic nerves stimulate bone marrow function after chemotherapy.Cancer Discov. 2013 Jul;3(7):OF15. doi: 10.1158/2159-8290.CD-RW2013-103. Epub 2013 May 16. Cancer Discov. 2013. PMID: 23847358
-
Electrical Sympathetic Neuromodulation Protects Bone Marrow Niche and Drives Hematopoietic Regeneration during Chemotherapy.Small Methods. 2023 Jun;7(6):e2201300. doi: 10.1002/smtd.202201300. Epub 2023 Feb 26. Small Methods. 2023. PMID: 36843214
-
Adrenergic Modulation of Hematopoiesis.J Neuroimmune Pharmacol. 2020 Mar;15(1):82-92. doi: 10.1007/s11481-019-09840-7. Epub 2019 Feb 14. J Neuroimmune Pharmacol. 2020. PMID: 30762159 Review.
-
Hematopoietic Stem Cell and Its Bone Marrow Niche.Curr Top Dev Biol. 2016;118:21-44. doi: 10.1016/bs.ctdb.2016.01.009. Epub 2016 Mar 21. Curr Top Dev Biol. 2016. PMID: 27137653 Free PMC article. Review.
Cited by
-
Systemic and local regulation of hematopoietic homeostasis in health and disease.Nat Cardiovasc Res. 2024 Jun;3(6):651-665. doi: 10.1038/s44161-024-00482-4. Epub 2024 Jun 12. Nat Cardiovasc Res. 2024. PMID: 39196230 Review.
-
Macrophage Dysregulation and Impaired Skin Wound Healing in Diabetes.Front Cell Dev Biol. 2020 Jun 26;8:528. doi: 10.3389/fcell.2020.00528. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32671072 Free PMC article. Review.
-
Isoprenaline protects intestinal stem cells from chemotherapy-induced damage.Br J Pharmacol. 2020 Feb;177(3):687-700. doi: 10.1111/bph.14883. Epub 2020 Jan 3. Br J Pharmacol. 2020. PMID: 31648381 Free PMC article.
-
The cellular composition and function of the bone marrow niche after allogeneic hematopoietic cell transplantation.Bone Marrow Transplant. 2022 Sep;57(9):1357-1364. doi: 10.1038/s41409-022-01728-0. Epub 2022 Jun 11. Bone Marrow Transplant. 2022. PMID: 35690693 Free PMC article. Review.
-
Stromal STAT5-Mediated Trophic Activity Regulates Hematopoietic Niche Factors.Stem Cells. 2023 Oct 8;41(10):944-957. doi: 10.1093/stmcls/sxad055. Stem Cells. 2023. PMID: 37465968 Free PMC article.
References
-
- Banfi A, et al. High-dose chemotherapy shows a dose-dependent toxicity to bone marrow osteoprogenitors: a mechanism for post-bone marrow transplantation osteopenia. Cancer. 2001;92:2419–2428. - PubMed
-
- Mauch P, et al. Hematopoietic stem cell compartment: acute and late effects of radiation therapy and chemotherapy. Int J Radiat Oncol Biol Phys. 1995;31:1319–1339. - PubMed
-
- Haas R, et al. Patient characteristics associated with successful mobilizing and autografting of peripheral blood progenitor cells in malignant lymphoma. Blood. 1994;83:3787–3794. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

