Annexin peptide Ac2-26 suppresses TNFα-induced inflammatory responses via inhibition of Rac1-dependent NADPH oxidase in human endothelial cells
- PMID: 23637767
- PMCID: PMC3634803
- DOI: 10.1371/journal.pone.0060790
Annexin peptide Ac2-26 suppresses TNFα-induced inflammatory responses via inhibition of Rac1-dependent NADPH oxidase in human endothelial cells
Abstract
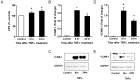
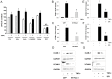
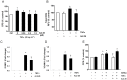
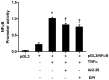
The anti-inflammatory peptide annexin-1 binds to formyl peptide receptors (FPR) but little is known about its mechanism of action in the vasculature. Here we investigate the effect of annexin peptide Ac2-26 on NADPH oxidase activity induced by tumour necrosis factor alpha (TNFα) in human endothelial cells. Superoxide release and intracellular reactive oxygen species (ROS) production from NADPH oxidase was measured with lucigenin-enhanced chemiluminescence and 2',7'-dichlorodihydrofluorescein diacetate, respectively. Expression of NADPH oxidase subunits and intracellular cell adhesion molecule (ICAM-1) and vascular cell adhesion molecule (VCAM-1) were determined by real-time PCR and Western blot analysis. Promoter activity of nuclear factor kappa B (NFκB) was measured by luciferase activity assay. TNFα stimulated NADPH-dependent superoxide release, total ROS formation and expression of ICAM-1and VCAM-1. Pre-treatment with N-terminal peptide of annexin-1 (Ac2-26, 0.5-1.5 µM) reduced all these effects, and the inhibition was blocked by the FPRL-1 antagonist WRW4. Furthermore, TNFα-induced NFκB promoter activity was attenuated by both Ac2-26 and NADPH oxidase inhibitor diphenyliodonium (DPI). Surprisingly, Nox4 gene expression was reduced by TNFα whilst expression of Nox2, p22phox and p67phox remained unchanged. Inhibition of NADPH oxidase activity by either dominant negative Rac1 (N17Rac1) or DPI significantly attenuated TNFα-induced ICAM-1and VCAM-1 expression. Ac2-26 failed to suppress further TNFα-induced expression of ICAM-1 and VCAM-1 in N17Rac1-transfected cells. Thus, Ac2-26 peptide inhibits TNFα-activated, Rac1-dependent NADPH oxidase derived ROS formation, attenuates NFκB pathways and ICAM-1 and VCAM-1 expression in endothelial cells. This suggests that Ac2-26 peptide blocks NADPH oxidase activity and has anti-inflammatory properties in the vasculature which contributes to modulate in reperfusion injury inflammation and vascular disease.
Conflict of interest statement
Figures

Similar articles
-
NF- kappa B independent suppression of endothelial vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 gene expression by inhibition of flavin binding proteins and superoxide production.J Mol Cell Cardiol. 2000 Aug;32(8):1499-508. doi: 10.1006/jmcc.2000.1183. J Mol Cell Cardiol. 2000. PMID: 10900176
-
Rac1 and superoxide are required for the expression of cell adhesion molecules induced by tumor necrosis factor-alpha in endothelial cells.J Pharmacol Exp Ther. 2003 May;305(2):573-80. doi: 10.1124/jpet.102.047894. Epub 2003 Feb 11. J Pharmacol Exp Ther. 2003. PMID: 12606638
-
Clematichinenoside inhibits VCAM-1 and ICAM-1 expression in TNF-α-treated endothelial cells via NADPH oxidase-dependent IκB kinase/NF-κB pathway.Free Radic Biol Med. 2015 Jan;78:190-201. doi: 10.1016/j.freeradbiomed.2014.11.004. Epub 2014 Nov 13. Free Radic Biol Med. 2015. PMID: 25463279
-
Vascular cell adhesion molecule-1 expression and signaling during disease: regulation by reactive oxygen species and antioxidants.Antioxid Redox Signal. 2011 Sep 15;15(6):1607-38. doi: 10.1089/ars.2010.3522. Epub 2011 May 11. Antioxid Redox Signal. 2011. PMID: 21050132 Free PMC article. Review.
-
Towards specific NADPH oxidase inhibition by small synthetic peptides.Cell Mol Life Sci. 2012 Jul;69(14):2307-14. doi: 10.1007/s00018-012-1008-3. Epub 2012 May 6. Cell Mol Life Sci. 2012. PMID: 22562604 Free PMC article. Review.
Cited by
-
Lipoxin A4 attenuates uric acid-activated, NADPH oxidase-dependent oxidative stress by interfering with translocation of p47phox in human umbilical vein endothelial cells.Exp Ther Med. 2020 Aug;20(2):1682-1692. doi: 10.3892/etm.2020.8812. Epub 2020 May 28. Exp Ther Med. 2020. PMID: 32765680 Free PMC article.
-
Pericardial Fluid Annexin A1 Is a Marker of Atrial Fibrillation in Aortic Stenosis: A Proteomics Analysis.J Pers Med. 2022 Feb 11;12(2):264. doi: 10.3390/jpm12020264. J Pers Med. 2022. PMID: 35207752 Free PMC article.
-
Rap1 GTPase Inhibits Tumor Necrosis Factor-α-Induced Choroidal Endothelial Migration via NADPH Oxidase- and NF-κB-Dependent Activation of Rac1.Am J Pathol. 2015 Dec;185(12):3316-25. doi: 10.1016/j.ajpath.2015.08.017. Epub 2015 Oct 23. Am J Pathol. 2015. PMID: 26476350 Free PMC article.
-
The role and mechanism of the annexin A1 peptide Ac2-26 in rats with cardiopulmonary bypass lung injury.Basic Clin Pharmacol Toxicol. 2021 Jun;128(6):719-730. doi: 10.1111/bcpt.13561. Epub 2021 Feb 1. Basic Clin Pharmacol Toxicol. 2021. PMID: 33455036 Free PMC article.
-
The Novel Small-molecule Annexin-A1 Mimetic, Compound 17b, Elicits Vasoprotective Actions in Streptozotocin-induced Diabetic Mice.Int J Mol Sci. 2020 Feb 18;21(4):1384. doi: 10.3390/ijms21041384. Int J Mol Sci. 2020. PMID: 32085666 Free PMC article.
References
-
- Gerke V, Moss SE (2002) Annexins: from structure to function. Physiol Rev 82: 331–371. - PubMed
-
- Fan J, Frey RS, Rahman A, Malik AB (2002) Role of neutrophil NADPH oxidase in the mechanism of tumor necrosis factor-alpha -induced NF-kappa B activation and intercellular adhesion molecule-1 expression in endothelial cells. J Biol Chem 277: 3404–3411. - PubMed
-
- Gavins FN, Dalli J, Flower RJ, Granger DN, Perretti M (2007) Activation of the annexin 1 counter-regulatory circuit affords protection in the mouse brain microcirculation. Faseb J 21: 1751–1758. - PubMed
-
- Perretti M, Croxtall JD, Wheller SK, Goulding NJ, Hannon R, et al. (1996) Mobilizing lipocortin 1 in adherent human leukocytes downregulates their transmigration. Nat Med 2: 1259–1262. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

