Human cytomegalovirus-induced NKG2C(hi) CD57(hi) natural killer cells are effectors dependent on humoral antiviral immunity
- PMID: 23637420
- PMCID: PMC3700275
- DOI: 10.1128/JVI.01096-13
Human cytomegalovirus-induced NKG2C(hi) CD57(hi) natural killer cells are effectors dependent on humoral antiviral immunity
Abstract
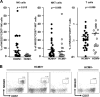
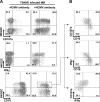
Recent studies indicate that expansion of NKG2C-positive natural killer (NK) cells is associated with human cytomegalovirus (HCMV); however, their activity in response to HCMV-infected cells remains unclear. We show that NKG2C(hi) CD57(hi) NK cells gated on CD3(neg) CD56(dim) cells can be phenotypically identified as HCMV-induced NK cells that can be activated by HCMV-infected cells. Using HCMV-infected autologous macrophages as targets, we were able to show that these NKG2C(hi) CD57(hi) NK cells are highly responsive to HCMV-infected macrophages only in the presence of HCMV-specific antibodies, whereas they are functionally poor effectors of natural cytotoxicity. We further demonstrate that NKG2C(hi) CD57(hi) NK cells are intrinsically responsive to signaling through CD16 cross-linking. Our findings show that the activity of pathogen-induced innate immune cells can be enhanced by adaptive humoral immunity. Understanding the activity of NKG2C(hi) CD57(hi) NK cells against HCMV-infected cells will be of relevance for the further development of adoptive immunotherapy.
Figures



Similar articles
-
Expansion of a unique CD57⁺NKG2Chi natural killer cell subset during acute human cytomegalovirus infection.Proc Natl Acad Sci U S A. 2011 Sep 6;108(36):14725-32. doi: 10.1073/pnas.1110900108. Epub 2011 Aug 8. Proc Natl Acad Sci U S A. 2011. PMID: 21825173 Free PMC article.
-
NKG2C(+)CD57(+) Natural Killer Cell Expansion Parallels Cytomegalovirus-Specific CD8(+) T Cell Evolution towards Senescence.J Immunol Res. 2016;2016:7470124. doi: 10.1155/2016/7470124. Epub 2016 May 29. J Immunol Res. 2016. PMID: 27314055 Free PMC article.
-
Cytomegalovirus-Driven Adaption of Natural Killer Cells in NKG2Cnull Human Immunodeficiency Virus-Infected Individuals.Viruses. 2019 Mar 9;11(3):239. doi: 10.3390/v11030239. Viruses. 2019. PMID: 30857329 Free PMC article.
-
The CD94/NKG2C+ NK-cell subset on the edge of innate and adaptive immunity to human cytomegalovirus infection.Semin Immunol. 2014 Apr;26(2):145-51. doi: 10.1016/j.smim.2014.03.002. Epub 2014 Mar 22. Semin Immunol. 2014. PMID: 24666761 Review.
-
Adaptive reconfiguration of the human NK-cell compartment in response to cytomegalovirus: a different perspective of the host-pathogen interaction.Eur J Immunol. 2013 May;43(5):1133-41. doi: 10.1002/eji.201243117. Eur J Immunol. 2013. PMID: 23552990 Review.
Cited by
-
Impaired T cells and "memory-like" NK-cell reconstitution is linked to late-onset HCMV reactivation after letermovir cessation.Blood Adv. 2024 Jun 11;8(11):2967-2979. doi: 10.1182/bloodadvances.2023012008. Blood Adv. 2024. PMID: 38315873 Free PMC article.
-
CD2 Immunobiology.Front Immunol. 2020 Jun 9;11:1090. doi: 10.3389/fimmu.2020.01090. eCollection 2020. Front Immunol. 2020. PMID: 32582179 Free PMC article. Review.
-
Functional Significance of CD57 Expression on Human NK Cells and Relevance to Disease.Front Immunol. 2013 Dec 9;4:422. doi: 10.3389/fimmu.2013.00422. Front Immunol. 2013. PMID: 24367364 Free PMC article. Review.
-
Elusive Role of the CD94/NKG2C NK Cell Receptor in the Response to Cytomegalovirus: Novel Experimental Observations in a Reporter Cell System.Front Immunol. 2017 Oct 24;8:1317. doi: 10.3389/fimmu.2017.01317. eCollection 2017. Front Immunol. 2017. PMID: 29114247 Free PMC article.
-
High frequencies of adaptive NK cells are associated with absence of coronary plaque in cytomegalovirus infected people living with HIV.Medicine (Baltimore). 2022 Sep 23;101(38):e30794. doi: 10.1097/MD.0000000000030794. Medicine (Baltimore). 2022. PMID: 36197157 Free PMC article.
References
-
- Lee SH, Miyagi T, Biron CA. 2007. Keeping NK cells in highly regulated antiviral warfare. Trends Immunol. 28:252–259 - PubMed
-
- Biron CA, Byron KS, Sullivan JL. 1989. Severe herpesvirus infections in an adolescent without natural killer cells. N. Engl. J. Med. 320:1731–1735 - PubMed
-
- Kuijpers TW, Baars PA, Dantin C, van den Burg M, van Lier RA, Roosnek E. 2008. Human NK cells can control CMV infection in the absence of T cells. Blood 112:914–915 - PubMed
-
- Hertenstein B, Hampl W, Bunjes D, Wiesneth M, Duncker C, Koszinowski U, Heimpel H, Arnold R, Mertens T. 1995. In vivo/ex vivo T cell depletion for GVHD prophylaxis influences onset and course of active cytomegalovirus infection and disease after BMT. Bone Marrow Transplant. 15:387–393 - PubMed
-
- Venema H, van den Berg AP, van Zanten C, van Son WJ, van der Giessen M, The TH. 1994. Natural killer cell responses in renal transplant patients with cytomegalovirus infection. J. Med. Virol. 42:188–192 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

