miR-124 inhibits STAT3 signaling to enhance T cell-mediated immune clearance of glioma
- PMID: 23636127
- PMCID: PMC3780786
- DOI: 10.1158/0008-5472.CAN-12-4318
miR-124 inhibits STAT3 signaling to enhance T cell-mediated immune clearance of glioma
Abstract
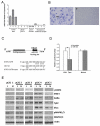
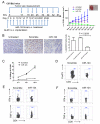
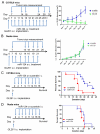
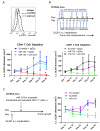
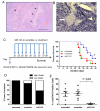
miRNAs (miR) have been shown to modulate critical gene transcripts involved in tumorigenesis, but their role in tumor-mediated immunosuppression is largely unknown. On the basis of miRNA gene expression in gliomas using tissue microarrays, in situ hybridization, and molecular modeling, miR-124 was identified as a lead candidate for modulating STAT3 signaling, a key pathway mediating immunosuppression in the tumor microenvironment. miR-124 is absent in all grades and pathologic types of gliomas. Upon upregulating miR-124 in glioma cancer stem cells (gCSC), the STAT3 pathway was inhibited, and miR-124 reversed gCSC-mediated immunosuppression of T-cell proliferation and induction of forkhead box P3 (Foxp3)(+) regulatory T cells (Treg). Treatment of T cells from immunosuppressed glioblastoma patients with miR-124 induced marked effector response including upregulation of interleukin (IL)-2, IFN-γ, and TNF-α. Both systemic administration of miR-124 or adoptive miR-124-transfected T-cell transfers exerted potent anti-glioma therapeutic effects in clonotypic and genetically engineered murine models of glioblastoma and enhanced effector responses in the local tumor microenvironment. These therapeutic effects were ablated in both CD4(+)- and CD8(+)-depleted mice and nude mouse systems, indicating that the therapeutic effect of miR-124 depends on the presence of a T-cell-mediated antitumor immune response. Our findings highlight the potential application of miR-124 as a novel immunotherapeutic agent for neoplasms and serve as a model for identifying miRNAs that can be exploited as immunotherapeutics.
©2013 AACR.
Figures

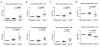
Similar articles
-
MiR-138 exerts anti-glioma efficacy by targeting immune checkpoints.Neuro Oncol. 2016 May;18(5):639-48. doi: 10.1093/neuonc/nov292. Epub 2015 Dec 11. Neuro Oncol. 2016. PMID: 26658052 Free PMC article.
-
B7-H4(B7x)-Mediated Cross-talk between Glioma-Initiating Cells and Macrophages via the IL6/JAK/STAT3 Pathway Lead to Poor Prognosis in Glioma Patients.Clin Cancer Res. 2016 Jun 1;22(11):2778-2790. doi: 10.1158/1078-0432.CCR-15-0858. Epub 2016 Mar 21. Clin Cancer Res. 2016. PMID: 27001312 Free PMC article.
-
MiR-24 regulates the proliferation and invasion of glioma by ST7L via β-catenin/Tcf-4 signaling.Cancer Lett. 2013 Feb 28;329(2):174-80. doi: 10.1016/j.canlet.2012.10.025. Epub 2012 Nov 7. Cancer Lett. 2013. PMID: 23142218
-
Regulation of the MIR155 host gene in physiological and pathological processes.Gene. 2013 Dec 10;532(1):1-12. doi: 10.1016/j.gene.2012.12.009. Epub 2012 Dec 14. Gene. 2013. PMID: 23246696 Review.
-
Targeting strategies on miRNA-21 and PDCD4 for glioblastoma.Arch Biochem Biophys. 2015 Aug 15;580:64-74. doi: 10.1016/j.abb.2015.07.001. Epub 2015 Jul 2. Arch Biochem Biophys. 2015. PMID: 26142886 Review.
Cited by
-
Exploring the clinical implications and applications of exosomal miRNAs in gliomas: a comprehensive study.Cancer Cell Int. 2024 Sep 27;24(1):323. doi: 10.1186/s12935-024-03507-x. Cancer Cell Int. 2024. PMID: 39334350 Free PMC article. Review.
-
miRNAs in radiotherapy resistance of cancer; a comprehensive review.Cell Biochem Biophys. 2024 Sep;82(3):1665-1679. doi: 10.1007/s12013-024-01329-2. Epub 2024 May 28. Cell Biochem Biophys. 2024. PMID: 38805114 Review.
-
The role of miRNAs in T helper cell development, activation, fate decisions and tumor immunity.Front Immunol. 2024 Jan 9;14:1320305. doi: 10.3389/fimmu.2023.1320305. eCollection 2023. Front Immunol. 2024. PMID: 38264670 Free PMC article. Review.
-
Tumor-specific polycistronic miRNA delivered by engineered exosomes for the treatment of glioblastoma.Neuro Oncol. 2024 Feb 2;26(2):236-250. doi: 10.1093/neuonc/noad199. Neuro Oncol. 2024. PMID: 37847405 Free PMC article.
-
Non-coding RNA in tumor-infiltrating regulatory T cells formation and associated immunotherapy.Front Immunol. 2023 Aug 21;14:1228331. doi: 10.3389/fimmu.2023.1228331. eCollection 2023. Front Immunol. 2023. PMID: 37671150 Free PMC article. Review.
References
-
- Brooks WH, Markesbery WR, Gupta GD, Roszman TL. Relationship of lymphocyte invasion and survival of brain tumor patients. Ann Neurol. 1978;4:219–24. - PubMed
-
- Jacobs JF, Idema AJ, Bol KF, Grotenhuis JA, de Vries IJ, Wesseling P, et al. Prognostic significance and mechanism of Treg infiltration in human brain tumors. J Neuroimmunol. 2010;225:195–9. - PubMed
-
- Palma L, Di Lorenzo N, Guidetti B. Lymphocytic infiltrates in primary glioblastomas and recidivous gliomas. Incidence, fate, and relevance to prognosis in 228 operated cases. J Neurosurg. 1978;49:854–61. - PubMed
-
- Safdari H, Hochberg FH, Richardson EP., Jr Prognostic value of round cell (lymphocyte) infiltration in malignant gliomas. Surg Neurol. 1985;23:221–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

