Combination neratinib (HKI-272) and paclitaxel therapy in patients with HER2-positive metastatic breast cancer
- PMID: 23632474
- PMCID: PMC3670493
- DOI: 10.1038/bjc.2013.178
Combination neratinib (HKI-272) and paclitaxel therapy in patients with HER2-positive metastatic breast cancer
Abstract
Introduction: Neratinib is a potent irreversible pan-ErbB tyrosine kinase inhibitor that has demonstrated antitumour activity and an acceptable safety profile in patients with human epidermal growth factor receptor (HER)-2-positive breast cancer and other solid tumours.
Methods: This was a phase I/II, open-label, two-part study. Part 1 was a dose-escalation study to determine the maximum tolerated dose (MTD) of neratinib plus paclitaxel in patients with solid tumours. Part 2 evaluated the safety, efficacy, and pharmacokinetics of the combination at the MTD in patients with HER2-positive breast cancer.
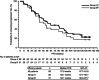
Results: Eight patients were included in the dose-escalation study; no dose-limiting toxicities were observed, and an MTD of oral neratinib 240 mg once daily plus intravenous paclitaxel 80 mg m(-2) on days 1, 8, and 15 of each 28-day cycle was determined. A total of 102 patients with HER2-positive breast cancer were enrolled in part 2. The overall median treatment duration was 47.9 weeks (range: 0.1-147.3 weeks). Common treatment-emergent adverse events (all grades/grade ≥3) included diarrhoea (92%/29%; none grade 4), peripheral sensory neuropathy (51%/3%), neutropenia (50%/20%), alopecia (46%/0%), leukopenia (41%/18%), anaemia (37%/8%), and nausea (34%/1%). Three (3%) patients discontinued treatment due to an adverse event (mouth ulceration, left ventricular ejection fraction reduction, and acute renal failure). Among the 99 evaluable patients in part 2 of the study, the overall response rate (ORR) was 73% (95% confidence interval (CI): 62.9-81.2%), including 7 (7%) patients who achieved a complete response; an additional 9 (9%) patients achieved stable disease for at least 24 weeks. ORR was 71% among patients with 0/1 prior chemotherapy regimen for metastatic disease and no prior lapatinib, and 77% among those with 2/3 prior chemotherapy regimens for metastatic disease with prior lapatinib permitted. Kaplan-Meier median progression-free survival was 57.0 weeks (95% CI: 47.7-81.6 weeks). Pharmacokinetic analyses indicated no interaction between neratinib and paclitaxel.
Conclusion: The combination of neratinib and paclitaxel was associated with higher toxicity than that of neratinib as a single agent, but was manageable with antidiarrhoeal agents and dose reductions in general. The combination therapy also demonstrated a high rate of response in patients with HER2-positive breast cancer. A phase III trial is ongoing to assess the benefit and risk of this combination in the first-line setting.
Trial registration: ClinicalTrials.gov NCT00445458.
Figures
Similar articles
-
Safety and efficacy of neratinib in combination with capecitabine in patients with metastatic human epidermal growth factor receptor 2-positive breast cancer.J Clin Oncol. 2014 Nov 10;32(32):3626-33. doi: 10.1200/JCO.2014.56.3809. Epub 2014 Oct 6. J Clin Oncol. 2014. PMID: 25287822 Clinical Trial.
-
Safety and efficacy of neratinib in combination with weekly paclitaxel and trastuzumab in women with metastatic HER2‑positive breast cancer: an NSABP Foundation Research Program phase I study.Cancer Chemother Pharmacol. 2013 Dec;72(6):1205-12. doi: 10.1007/s00280-013-2262-2. Cancer Chemother Pharmacol. 2013. PMID: 24077916 Clinical Trial.
-
Safety and efficacy of neratinib (HKI-272) plus vinorelbine in the treatment of patients with ErbB2-positive metastatic breast cancer pretreated with anti-HER2 therapy.Ann Oncol. 2013 Jan;24(1):109-16. doi: 10.1093/annonc/mds284. Epub 2012 Sep 11. Ann Oncol. 2013. PMID: 22967996 Clinical Trial.
-
The Clinical Efficacy and Safety of Neratinib in Combination with Capecitabine for the Treatment of Adult Patients with Advanced or Metastatic HER2-Positive Breast Cancer.Drug Des Devel Ther. 2021 Jun 21;15:2711-2720. doi: 10.2147/DDDT.S281599. eCollection 2021. Drug Des Devel Ther. 2021. PMID: 34188449 Free PMC article. Review.
-
Pharmacodynamics, pharmacokinetics and clinical efficacy of neratinib in HER2-positive breast cancer and breast cancer with HER2 mutations.Expert Opin Drug Metab Toxicol. 2016 Aug;12(8):947-57. doi: 10.1080/17425255.2016.1198317. Epub 2016 Jun 27. Expert Opin Drug Metab Toxicol. 2016. PMID: 27284682 Review.
Cited by
-
Targeted Therapies in Older Adults With Solid Tumors.J Clin Oncol. 2021 Jul 1;39(19):2128-2137. doi: 10.1200/JCO.21.00132. Epub 2021 May 27. J Clin Oncol. 2021. PMID: 34043448 Free PMC article. Review. No abstract available.
-
Adaptive Randomization of Neratinib in Early Breast Cancer.N Engl J Med. 2016 Jul 7;375(1):11-22. doi: 10.1056/NEJMoa1513750. N Engl J Med. 2016. PMID: 27406346 Free PMC article. Clinical Trial.
-
CNS metastases in breast cancer: old challenge, new frontiers.Clin Cancer Res. 2013 Dec 1;19(23):6404-18. doi: 10.1158/1078-0432.CCR-13-0790. Clin Cancer Res. 2013. PMID: 24298071 Free PMC article. Review.
-
HER2-Mutated Breast Cancer Responds to Treatment With Single-Agent Neratinib, a Second-Generation HER2/EGFR Tyrosine Kinase Inhibitor.J Natl Compr Canc Netw. 2015 Sep;13(9):1061-4. doi: 10.6004/jnccn.2015.0131. J Natl Compr Canc Netw. 2015. PMID: 26358790 Free PMC article.
-
Human epidermal growth factor receptor family-targeted therapies in the treatment of HER2-overexpressing breast cancer.Oncologist. 2014 Feb;19(2):135-50. doi: 10.1634/theoncologist.2013-0283. Epub 2014 Jan 16. Oncologist. 2014. PMID: 24436312 Free PMC article. Review.
References
-
- Andersson M, Lidbrink E, Bjerre K, Wist E, Enevoldsen K, Jensen AB, Karlsson P, Tange UB, Sørensen PG, Møller S, Bergh J, Langkjer ST. Phase III randomized study comparing docetaxel plus trastuzumab with vinorelbine plus trastuzumab as first-line therapy of metastatic or locally advanced human epidermal growth factor receptor 2-positive breast cancer: the HERNATA study. J Clin Oncol. 2011;29 (3:264–271. - PubMed
-
- Andre F, Campone M, O'Regan R, Manlius C, Massacesi C, Sahmoud T, Mukhopadhyay P, Soria JC, Naughton M, Hurvitz SA. Phase I study of everolimus plus weekly paclitaxel and trastuzumab in patients with metastatic breast cancer pretreated with trastuzumab. J Clin Oncol. 2010;28 (34:5110–5115. - PubMed
-
- Benson AB, 3rd, Ajani JA, Catalano RB, Engelking C, Kornblau SM, Martenson JA, Jr, McCallum R, Mitchell EP, O'Dorisio TM, Vokes EE, Wadler S. Recommended guidelines for the treatment of cancer treatment-induced diarrhea. J Clin Oncol. 2004;22 (14:2918–2926. - PubMed
-
- Blackwell KL, Burstein HJ, Storniolo AM, Rugo H, Sledge G, Koehler M, Ellis C, Casey M, Vukelja S, Bischoff J, Baselga J, O'Shaughnessy J. Randomized study of lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol. 2010;28 (7:1124–1130. - PubMed
-
- Burstein HJ, Keshaviah A, Baron AD, Hart RD, Lambert-Falls R, Marcom PK, Gelman R, Winer EP. Trastuzumab plus vinorelbine or taxane chemotherapy for HER2-overexpressing metastatic breast cancer: the trastuzumab and vinorelbine or taxane study. Cancer. 2007;110 (5:965–972. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

