Comparison of extravillous trophoblast cells derived from human embryonic stem cells and from first trimester human placentas
- PMID: 23631809
- PMCID: PMC3799850
- DOI: 10.1016/j.placenta.2013.03.016
Comparison of extravillous trophoblast cells derived from human embryonic stem cells and from first trimester human placentas
Abstract
Introduction: Preeclampsia and other placental pathologies are characterized by a lack of spiral artery remodeling associated with insufficient invasion by extravillous trophoblast cells (EVT). Because trophoblast invasion occurs in early pregnancy when access to human placental tissue is limited, there is a need for model systems for the study of trophoblast differentiation and invasion. Human embryonic stem cells (hESC) treated with BMP4- differentiate to trophoblast, and express HLA-G, a marker of EVT. The goals of the present study were to further characterize the HLA-G(+) cells derived from BMP4-treated hESC, and determine their suitability as a model.
Methods: HESC were treated with BMP4 under 4% or 20% oxygen and tested in Matrigel invasion chambers. Both BMP4-treated hESC and primary human placental cells were separated into HLA-G(+) and HLA-G(-)/TACSTD2(+) populations with immunomagnetic beads and expression profiles analyzed by microarray.
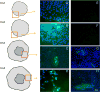
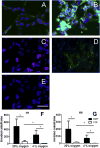
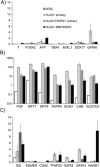
Results: There was a 10-fold increase in invasion when hESC were BMP4-treated. There was also an independent, stimulatory effect of oxygen on this process. Invasive cells expressed trophoblast marker KRT7, and the majority were also HLA-G(+). Gene expression profiles revealed that HLA-G(+), BMP4-treated hESC were similar to, but distinct from, HLA-G(+) cells isolated from first trimester placentas. Whereas HLA-G(+) and HLA-G(-) cells from first trimester placentas had highly divergent gene expression profiles, HLA-G(+) and HLA-G(-) cells from BMP4-treated hESC had somewhat similar profiles, and both expressed genes characteristic of early trophoblast development.
Conclusions: We conclude that hESC treated with BMP4 provide a model for studying transition to the EVT lineage.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures


Similar articles
-
BMP4-directed trophoblast differentiation of human embryonic stem cells is mediated through a ΔNp63+ cytotrophoblast stem cell state.Development. 2013 Oct;140(19):3965-76. doi: 10.1242/dev.092155. Epub 2013 Sep 4. Development. 2013. PMID: 24004950 Free PMC article.
-
Complete and unidirectional conversion of human embryonic stem cells to trophoblast by BMP4.Proc Natl Acad Sci U S A. 2013 Mar 26;110(13):E1212-21. doi: 10.1073/pnas.1303094110. Epub 2013 Mar 14. Proc Natl Acad Sci U S A. 2013. PMID: 23493551 Free PMC article.
-
Cyclosporin A Promotes Invasion and Migration of Extravillous Trophoblast Cells Derived from Human-Induced Pluripotent Stem Cells and Human Embryonic Stem Cells.Stem Cells Dev. 2023 Feb;32(3-4):60-74. doi: 10.1089/scd.2022.0144. Stem Cells Dev. 2023. PMID: 36476041
-
Investigation of human trophoblast invasion in vitro.Hum Reprod Update. 2020 Jun 18;26(4):501-513. doi: 10.1093/humupd/dmaa017. Hum Reprod Update. 2020. PMID: 32441309 Free PMC article. Review.
-
Trophoblast retrieval and isolation from the cervix: origins of cervical trophoblasts and their potential value for risk assessment of ongoing pregnancies.Hum Reprod Update. 2018 Jul 1;24(4):484-496. doi: 10.1093/humupd/dmy008. Hum Reprod Update. 2018. PMID: 29608700 Free PMC article. Review.
Cited by
-
SCDevDB: A Database for Insights Into Single-Cell Gene Expression Profiles During Human Developmental Processes.Front Genet. 2019 Sep 26;10:903. doi: 10.3389/fgene.2019.00903. eCollection 2019. Front Genet. 2019. PMID: 31611909 Free PMC article.
-
MicroRNA-590-3p inhibits trophoblast-dependent maternal spiral artery remodeling by repressing low-density lipoprotein receptor-related protein 6.Mol Genet Genomic Med. 2018 Nov;6(6):1124-1133. doi: 10.1002/mgg3.491. Epub 2018 Nov 8. Mol Genet Genomic Med. 2018. PMID: 30411539 Free PMC article.
-
What Is Trophoblast? A Combination of Criteria Define Human First-Trimester Trophoblast.Stem Cell Reports. 2016 Feb 9;6(2):257-72. doi: 10.1016/j.stemcr.2016.01.006. Stem Cell Reports. 2016. PMID: 26862703 Free PMC article.
-
Single-cell transcriptional profiling reveals cellular and molecular divergence in human maternal-fetal interface.Sci Rep. 2022 Jun 28;12(1):10892. doi: 10.1038/s41598-022-14516-z. Sci Rep. 2022. PMID: 35764880 Free PMC article.
-
Human HLA-G+ extravillous trophoblasts: Immune-activating cells that interact with decidual leukocytes.Proc Natl Acad Sci U S A. 2015 Jun 9;112(23):7219-24. doi: 10.1073/pnas.1507977112. Epub 2015 May 26. Proc Natl Acad Sci U S A. 2015. PMID: 26015573 Free PMC article.
References
-
- Aplin JD. Developmental cell biology of human villous trophoblast: current research problems. Int J Dev Biol. 2010;54(2–3):323–9. - PubMed
-
- Caniggia I, Winter J, Lye SJ, Post M. Oxygen and placental development during the first trimester: implications for the pathophysiology of pre-eclampsia. Placenta. 2000;21(Suppl A):S25–30. - PubMed
-
- Kaufmann P, Black S, Huppertz B. Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and pre-eclampsia. Biol Reprod. 2003;69(1):1–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

