Tubulin polymerization-promoting protein (TPPP/p25α) promotes unconventional secretion of α-synuclein through exophagy by impairing autophagosome-lysosome fusion
- PMID: 23629650
- PMCID: PMC3682534
- DOI: 10.1074/jbc.M112.401174
Tubulin polymerization-promoting protein (TPPP/p25α) promotes unconventional secretion of α-synuclein through exophagy by impairing autophagosome-lysosome fusion
Abstract
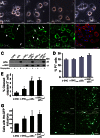
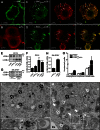
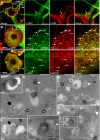
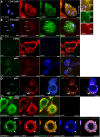
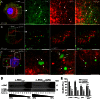
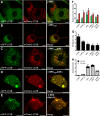
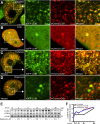
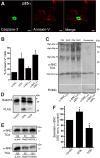
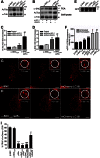
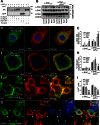
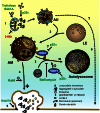
Aggregation of α-synuclein can be promoted by the tubulin polymerization-promoting protein/p25α, which we have used here as a tool to study the role of autophagy in the clearance of α-synuclein. In NGF-differentiated PC12 catecholaminergic nerve cells, we show that de novo expressed p25α co-localizes with α-synuclein and causes its aggregation and distribution into autophagosomes. However, p25α also lowered the mobility of autophagosomes and hindered the final maturation of autophagosomes by preventing their fusion with lysosomes for the final degradation of α-synuclein. Instead, p25α caused a 4-fold increase in the basal level of α-synuclein secreted into the medium. Secretion was strictly dependent on autophagy and could be up-regulated (trehalose and Rab1A) or down-regulated (3-methyladenine and ATG5 shRNA) by enhancers or inhibitors of autophagy or by modulating minus-end-directed (HDAC6 shRNA) or plus-end-directed (Rab8) trafficking of autophagosomes along microtubules. Finally, we show in the absence of tubulin polymerization-promoting protein/p25α that α-synuclein release was modulated by dominant mutants of Rab27A, known to regulate exocytosis of late endosomal (and amphisomal) elements, and that both lysosomal fusion block and secretion of α-synuclein could be replicated by knockdown of the p25α target, HDAC6, the predominant cytosolic deacetylase in neurons. Our data indicate that unconventional secretion of α-synuclein can be mediated through exophagy and that factors, which increase the pool of autophagosomes/amphisomes (e.g. lysosomal disturbance) or alter the polarity of vesicular transport of autophagosomes on microtubules, can result in an increased release of α-synuclein monomer and aggregates to the surroundings.
Keywords: Autophagosomes; Exophagy; HDAC6; Parkinson Disease; Protein Degradation; Protein Secretion; Trafficking; p25α; α-Synuclein.
Figures

Similar articles
-
α-synuclein buildup is alleviated via ESCRT-dependent endosomal degradation brought about by p38MAPK inhibition in cells expressing p25α.J Biol Chem. 2022 Nov;298(11):102531. doi: 10.1016/j.jbc.2022.102531. Epub 2022 Sep 24. J Biol Chem. 2022. PMID: 36162505 Free PMC article.
-
Reciprocal signals between microglia and neurons regulate α-synuclein secretion by exophagy through a neuronal cJUN-N-terminal kinase-signaling axis.J Neuroinflammation. 2016 Mar 8;13(1):59. doi: 10.1186/s12974-016-0519-5. J Neuroinflammation. 2016. PMID: 26957005 Free PMC article.
-
Alpha-Synuclein defects autophagy by impairing SNAP29-mediated autophagosome-lysosome fusion.Cell Death Dis. 2021 Sep 17;12(10):854. doi: 10.1038/s41419-021-04138-0. Cell Death Dis. 2021. PMID: 34535638 Free PMC article.
-
Prelysosomal Compartments in the Unconventional Secretion of Amyloidogenic Seeds.Int J Mol Sci. 2017 Jan 23;18(1):227. doi: 10.3390/ijms18010227. Int J Mol Sci. 2017. PMID: 28124989 Free PMC article. Review.
-
Biological Roles of Alternative Autophagy.Mol Cells. 2018 Jan 31;41(1):50-54. doi: 10.14348/molcells.2018.2215. Epub 2018 Jan 23. Mol Cells. 2018. PMID: 29370693 Free PMC article. Review.
Cited by
-
Emerging role of microglia in inter-cellular transmission of α-synuclein in Parkinson's disease.Front Aging Neurosci. 2024 Oct 9;16:1411104. doi: 10.3389/fnagi.2024.1411104. eCollection 2024. Front Aging Neurosci. 2024. PMID: 39444806 Free PMC article. Review.
-
Histone Deacetylase 6 and the Disease Mechanisms of α-Synucleinopathies.Front Synaptic Neurosci. 2020 Sep 11;12:586453. doi: 10.3389/fnsyn.2020.586453. eCollection 2020. Front Synaptic Neurosci. 2020. PMID: 33041780 Free PMC article. Review.
-
Exosomal cargos modulate autophagy in recipient cells via different signaling pathways.Cell Biosci. 2020 Aug 1;10:92. doi: 10.1186/s13578-020-00455-7. eCollection 2020. Cell Biosci. 2020. PMID: 32765827 Free PMC article. Review.
-
C. elegans Models to Study the Propagation of Prions and Prion-Like Proteins.Biomolecules. 2020 Aug 15;10(8):1188. doi: 10.3390/biom10081188. Biomolecules. 2020. PMID: 32824215 Free PMC article. Review.
-
Pathogenesis of α-Synuclein in Parkinson's Disease: From a Neuron-Glia Crosstalk Perspective.Int J Mol Sci. 2022 Nov 25;23(23):14753. doi: 10.3390/ijms232314753. Int J Mol Sci. 2022. PMID: 36499080 Free PMC article. Review.
References
-
- Braak H., Del Tredici K., Rüb U., de Vos R. A., Jansen Steur E. N., Braak E. (2003) Staging of brain pathology related to sporadic Parkinson disease. Neurobiol. Aging 24, 197–211 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

