Matrix metalloproteinases inhibition promotes the polyfunctionality of human natural killer cells in therapeutic antibody-based anti-tumour immunotherapy
- PMID: 23607800
- PMCID: PMC3694543
- DOI: 10.1111/cei.12095
Matrix metalloproteinases inhibition promotes the polyfunctionality of human natural killer cells in therapeutic antibody-based anti-tumour immunotherapy
Abstract
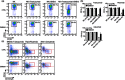
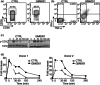
Activation of human natural killer (NK) cells is associated with the cleavage of CD16 from the cell surface, a process mediated by matrix metalloproteinases (MMPs). In this report, we examined whether inhibition of MMPs would lead to improved NK cell antibody-dependent cell-mediated cytotoxicity (ADCC) function. Using an in-vitro ADCC assay, we tested the anti-tumour function of NK cells with three different therapeutic monoclonal antibodies (mAbs) in the presence of MMPs inhibitor GM6001 or its control. Loss of CD16 was observed when NK cells were co-cultured with tumour targets in the presence of specific anti-tumour antibodies, and was found particularly on the majority of degranulating NK responding cells. Treatment with MMPs inhibitors not only prevented CD16 down-regulation, but improved the quality of the responding cells significantly, as shown by an increase in the percentage of polyfunctional NK cells that are capable of both producing cytokines and degranulation. Furthermore, MMPs inhibition resulted in augmented and sustained CD16-mediated signalling, as shown by increased tyrosine phosphorylation of CD3ζ and other downstream signalling intermediates, which may account for the improved NK cell function. Collectively, our results provide a foundation for combining MMPs inhibitors and therapeutic mAbs in new clinical trials for cancer treatment.
Published 2013. This article is a U.S. Government work and is in the public domain in the USA.
Figures

Similar articles
-
Effects of ADAM10 and ADAM17 Inhibitors on Natural Killer Cell Expansion and Antibody-dependent Cellular Cytotoxicity Against Breast Cancer Cells In Vitro.Anticancer Res. 2017 Oct;37(10):5507-5513. doi: 10.21873/anticanres.11981. Anticancer Res. 2017. PMID: 28982863
-
NK-cell activation and antibody-dependent cellular cytotoxicity induced by rituximab-coated target cells is inhibited by the C3b component of complement.Blood. 2008 Feb 1;111(3):1456-63. doi: 10.1182/blood-2007-02-074716. Epub 2007 Nov 16. Blood. 2008. PMID: 18024795 Free PMC article.
-
Membrane-type 6 matrix metalloproteinase regulates the activation-induced downmodulation of CD16 in human primary NK cells.J Immunol. 2013 Aug 15;191(4):1883-94. doi: 10.4049/jimmunol.1300313. Epub 2013 Jul 12. J Immunol. 2013. PMID: 23851692 Free PMC article.
-
Mechanisms of NK Cell Activation and Clinical Activity of the Therapeutic SLAMF7 Antibody, Elotuzumab in Multiple Myeloma.Front Immunol. 2018 Nov 5;9:2551. doi: 10.3389/fimmu.2018.02551. eCollection 2018. Front Immunol. 2018. PMID: 30455698 Free PMC article. Review.
-
Antibody-Dependent Cell-Mediated Cytotoxicity Through Natural Killer (NK) Cells: Unlocking NK Cells for Future Immunotherapy.Curr Pharm Biotechnol. 2022;23(4):552-578. doi: 10.2174/1389201022666210820093608. Curr Pharm Biotechnol. 2022. PMID: 34414871 Review.
Cited by
-
Implication of Interleukin-12/15/18 and Ruxolitinib in the Phenotype, Proliferation, and Polyfunctionality of Human Cytokine-Preactivated Natural Killer Cells.Front Immunol. 2018 Apr 16;9:737. doi: 10.3389/fimmu.2018.00737. eCollection 2018. Front Immunol. 2018. PMID: 29713323 Free PMC article.
-
Natural killer cell engagers: From bi-specific to tri-specific and tetra-specific engagers for enhanced cancer immunotherapy.Clin Transl Med. 2024 Nov;14(11):e70046. doi: 10.1002/ctm2.70046. Clin Transl Med. 2024. PMID: 39472273 Free PMC article. Review.
-
Expansion of CD16 positive and negative human NK cells in response to tumor stimulation.Eur J Immunol. 2014 May;44(5):1517-25. doi: 10.1002/eji.201344170. Epub 2014 Feb 20. Eur J Immunol. 2014. PMID: 24469995 Free PMC article.
-
Natural Killer Cell Adoptive Transfer Therapy: Exploiting the First Line of Defense Against Cancer.Cancer J. 2015 Nov-Dec;21(6):486-91. doi: 10.1097/PPO.0000000000000156. Cancer J. 2015. PMID: 26588681 Free PMC article. Review.
-
A NKp80-Based Identification Strategy Reveals that CD56neg NK Cells Are Not Completely Dysfunctional in Health and Disease.iScience. 2020 Jul 24;23(7):101298. doi: 10.1016/j.isci.2020.101298. Epub 2020 Jun 20. iScience. 2020. PMID: 32622268 Free PMC article.
References
-
- Jiang XR, Song A, Bergelson S, et al. Advances in the assessment and control of the effector functions of therapeutic antibodies. Nat Rev Drug Discov. 2011;10:101–111. - PubMed
-
- Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer. 2012;12:278–287. - PubMed
-
- Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–640. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

