p21 both attenuates and drives senescence and aging in BubR1 progeroid mice
- PMID: 23602569
- PMCID: PMC3785294
- DOI: 10.1016/j.celrep.2013.03.028
p21 both attenuates and drives senescence and aging in BubR1 progeroid mice
Abstract
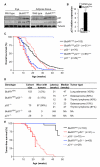
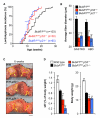
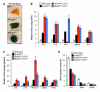
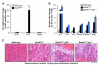
BubR1 insufficiency occurs with natural aging and induces progeroid phenotypes in both mice and children with mosaic variegated aneuploidy syndrome. In response to BubR1 insufficiency, skeletal muscle, fat, and lens tissue engage p19(Arf) to attenuate senescence and age-related deterioration. Here, we address how p19(Arf) exerts this caretaker role using BubR1 progeroid mice lacking p53 or its transcriptional target p21. We show that p53 delays functional decline of skeletal muscle and fat in a p21-dependent fashion by inhibiting p16(Ink4a)-mediated senescence of progenitor cells. Strikingly, p53 also attenuates the formation of cataractous lenses, but here its antiaging effect is p21 independent, as we found p21 to promote senescence of lens epithelial cells and cataract formation. Together, these results demonstrate that p53 counteracts tissue destruction in response to BubR1 insufficiency through diverse mechanisms and uncover a causal link between senescence of the progenitor cell compartment and age-related dysfunction.
Copyright © 2013 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Opposing roles for p16Ink4a and p19Arf in senescence and ageing caused by BubR1 insufficiency.Nat Cell Biol. 2008 Jul;10(7):825-36. doi: 10.1038/ncb1744. Epub 2008 May 30. Nat Cell Biol. 2008. PMID: 18516091 Free PMC article.
-
The yin and yang of the Cdkn2a locus in senescence and aging.Cell Cycle. 2008 Sep 15;7(18):2795-802. doi: 10.4161/cc.7.18.6687. Epub 2008 Sep 28. Cell Cycle. 2008. PMID: 18769141 Free PMC article.
-
Transcriptional coactivator with PDZ-binding motif is required to sustain testicular function on aging.Aging Cell. 2017 Oct;16(5):1035-1042. doi: 10.1111/acel.12631. Epub 2017 Jun 14. Aging Cell. 2017. PMID: 28613007 Free PMC article.
-
Resistance of primary cultured mouse hepatic tumor cells to cellular senescence despite expression of p16(Ink4a), p19(Arf), p53, and p21(Waf1/Cip1).Mol Carcinog. 2001 Sep;32(1):9-18. doi: 10.1002/mc.1059. Mol Carcinog. 2001. PMID: 11568971
-
Tumor suppressors and oncogenes in cellular senescence.Exp Gerontol. 2000 May;35(3):317-29. doi: 10.1016/s0531-5565(00)00083-8. Exp Gerontol. 2000. PMID: 10832053 Review.
Cited by
-
Cellular Senescence in Diabetes Mellitus: Distinct Senotherapeutic Strategies for Adipose Tissue and Pancreatic β Cells.Front Endocrinol (Lausanne). 2022 Mar 31;13:869414. doi: 10.3389/fendo.2022.869414. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35432205 Free PMC article. Review.
-
Modulation of Pancreatic Islets' Function and Survival During Aging Involves the Differential Regulation of Endoplasmic Reticulum Stress by p21 and CHOP.Antioxid Redox Signal. 2017 Aug 1;27(4):185-200. doi: 10.1089/ars.2016.6671. Epub 2017 Jan 16. Antioxid Redox Signal. 2017. PMID: 27931122 Free PMC article.
-
Senescent cells: an emerging target for diseases of ageing.Nat Rev Drug Discov. 2017 Oct;16(10):718-735. doi: 10.1038/nrd.2017.116. Epub 2017 Jul 21. Nat Rev Drug Discov. 2017. PMID: 28729727 Free PMC article. Review.
-
Senescence in Wound Repair: Emerging Strategies to Target Chronic Healing Wounds.Front Cell Dev Biol. 2020 Aug 11;8:773. doi: 10.3389/fcell.2020.00773. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32850866 Free PMC article. Review.
-
Aging of the Liver: What This Means for Patients with HIV.Curr HIV/AIDS Rep. 2016 Dec;13(6):309-317. doi: 10.1007/s11904-016-0332-x. Curr HIV/AIDS Rep. 2016. PMID: 27557619 Free PMC article. Review.
References
-
- Baker DJ, Jeganathan KB, Cameron JD, Thompson M, Juneja S, Kopecka A, Kumar R, Jenkins RB, de Groen PC, Roche P, van Deursen JM. BubR1 insufficiency causes early onset of aging-associated phenotypes and infertility in mice. Nat. Genet. 2004;36:744–749. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

