The ART-Rsp5 ubiquitin ligase network comprises a plasma membrane quality control system that protects yeast cells from proteotoxic stress
- PMID: 23599894
- PMCID: PMC3628405
- DOI: 10.7554/eLife.00459
The ART-Rsp5 ubiquitin ligase network comprises a plasma membrane quality control system that protects yeast cells from proteotoxic stress
Abstract
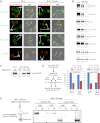
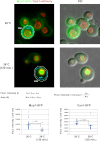
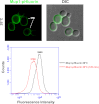
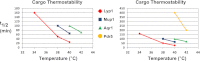
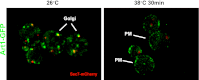
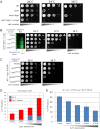
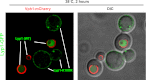
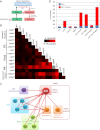
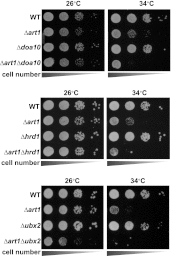
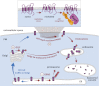
Secretory cargo that cannot fold properly in the ER are selectively targeted for removal by a well-studied ER-associated degradation pathway, or ERAD. In contrast, very little is known about post-ER quality control mechanisms for damaged or misfolded integral membrane proteins. Here we describe a quality control function of the Rsp5-ART ubiquitin ligase adaptor network that functions to protect plasma membrane (PM) integrity. Failure to mediate this protective response during heat stress leads to toxic accumulation of misfolded integral membrane proteins at the cell surface, which causes loss of PM integrity and cell death. Thus, the Rsp5-ART network comprises a PM quality control system that works together with sequential quality control pathways in the ER and Golgi to (i) target the degradation of proteins that have exceeded their functional lifetime due to damage and/or misfolding and (ii) limit the toxic accumulation of specific proteins at the cell surface during proteotoxic stress. DOI:http://dx.doi.org/10.7554/eLife.00459.001.
Keywords: S. cerevisiae; endocytosis; membrane traffic; proteostasis; quality control; ubiquitin.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures










Similar articles
-
Rsp5 Ubiquitin ligase-mediated quality control system clears membrane proteins mistargeted to the vacuole membrane.J Cell Biol. 2019 Jan 7;218(1):234-250. doi: 10.1083/jcb.201806094. Epub 2018 Oct 25. J Cell Biol. 2019. PMID: 30361468 Free PMC article.
-
Calcineurin-dependent regulation of endocytosis by a plasma membrane ubiquitin ligase adaptor, Rcr1.J Cell Biol. 2020 Aug 3;219(8):e201909158. doi: 10.1083/jcb.201909158. J Cell Biol. 2020. PMID: 32421152 Free PMC article.
-
Rsp5/Nedd4 is the main ubiquitin ligase that targets cytosolic misfolded proteins following heat stress.Nat Cell Biol. 2014 Dec;16(12):1227-37. doi: 10.1038/ncb3054. Epub 2014 Oct 26. Nat Cell Biol. 2014. PMID: 25344756 Free PMC article.
-
The ubiquitin code of yeast permease trafficking.Trends Cell Biol. 2010 Apr;20(4):196-204. doi: 10.1016/j.tcb.2010.01.004. Trends Cell Biol. 2010. PMID: 20138522 Review.
-
Role of Rsp5 ubiquitin ligase in biogenesis of rRNA, mRNA and tRNA in yeast.RNA Biol. 2015;12(12):1265-74. doi: 10.1080/15476286.2015.1094604. RNA Biol. 2015. PMID: 26403176 Free PMC article. Review.
Cited by
-
Endosome and Golgi-associated degradation (EGAD) of membrane proteins regulates sphingolipid metabolism.EMBO J. 2019 Aug 1;38(15):e101433. doi: 10.15252/embj.2018101433. Epub 2019 May 27. EMBO J. 2019. PMID: 31368600 Free PMC article.
-
Ubiquitin Ligase Redundancy and Nuclear-Cytoplasmic Localization in Yeast Protein Quality Control.Biomolecules. 2021 Dec 3;11(12):1821. doi: 10.3390/biom11121821. Biomolecules. 2021. PMID: 34944465 Free PMC article. Review.
-
Ubiquitin-dependent sorting in endocytosis.Cold Spring Harb Perspect Biol. 2014 Jan 1;6(1):a016808. doi: 10.1101/cshperspect.a016808. Cold Spring Harb Perspect Biol. 2014. PMID: 24384571 Free PMC article. Review.
-
Stress conditions promote yeast Gap1 permease ubiquitylation and down-regulation via the arrestin-like Bul and Aly proteins.J Biol Chem. 2014 Aug 8;289(32):22103-16. doi: 10.1074/jbc.M114.582320. Epub 2014 Jun 18. J Biol Chem. 2014. PMID: 24942738 Free PMC article.
-
Protein homeostasis at the plasma membrane.Physiology (Bethesda). 2014 Jul;29(4):265-77. doi: 10.1152/physiol.00058.2013. Physiology (Bethesda). 2014. PMID: 24985330 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

