Nucleolin inhibits Fas ligand binding and suppresses Fas-mediated apoptosis in vivo via a surface nucleolin-Fas complex
- PMID: 23599269
- PMCID: PMC3674671
- DOI: 10.1182/blood-2012-12-471094
Nucleolin inhibits Fas ligand binding and suppresses Fas-mediated apoptosis in vivo via a surface nucleolin-Fas complex
Abstract
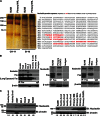
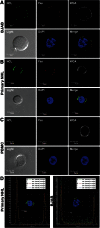
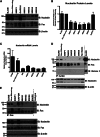
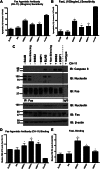
Resistance to Fas-mediated apoptosis is associated with poor cancer outcomes and chemoresistance. To elucidate potential mechanisms of defective Fas signaling, we screened primary lymphoma cell extracts for Fas-associated proteins that would have the potential to regulate Fas signaling. An activation-resistant Fas complex selectively included nucleolin. We confirmed the presence of nucleolin-Fas complexes in B-cell lymphoma cells and primary tissues, and the absence of such complexes in B-lymphocytes from healthy donors. RNA-binding domain 4 and the glycine/arginine-rich domain of nucleolin were essential for its association with Fas. Nucleolin colocalized with Fas on the surface of B-cell lymphoma cells. Nucleolin knockdown sensitized BJAB cells to Fas ligand (FasL)-induced and Fas agonistic antibody-induced apoptosis through enhanced binding, suggesting that nucleolin blocks the FasL-Fas interaction. Mice transfected with nucleolin were protected from the lethal effects of agonistic anti-mouse Fas antibody (Jo2) and had lower rates of hepatocyte apoptosis, compared with vector and a non-Fas-binding mutant of nucleolin. Our results show that cell surface nucleolin binds Fas, inhibits ligand binding, and thus prevents induction of Fas-mediated apoptosis in B-cell lymphomas and may serve as a new therapeutic target.
Figures

Similar articles
-
CD74 interferes with the expression of fas receptor on the surface of lymphoma cells.J Exp Clin Cancer Res. 2014 Oct 10;33(1):80. doi: 10.1186/s13046-014-0080-y. J Exp Clin Cancer Res. 2014. PMID: 25304249 Free PMC article.
-
Mechanism of Fas signaling regulation by human herpesvirus 8 K1 oncoprotein.J Natl Cancer Inst. 2009 Mar 18;101(6):399-411. doi: 10.1093/jnci/djn516. Epub 2009 Mar 10. J Natl Cancer Inst. 2009. PMID: 19276446 Free PMC article.
-
Hepatitis B virus core protein inhibits Fas-mediated apoptosis of hepatoma cells via regulation of mFas/FasL and sFas expression.FASEB J. 2015 Mar;29(3):1113-23. doi: 10.1096/fj.14-263822. Epub 2014 Dec 2. FASEB J. 2015. PMID: 25466893
-
Midkine, a cytokine that inhibits HIV infection by binding to the cell surface expressed nucleolin.Cell Res. 2006 Feb;16(2):174-81. doi: 10.1038/sj.cr.7310024. Cell Res. 2006. PMID: 16474431 Review.
-
B-cell receptor and Fas-mediated signals for life and death.Immunol Rev. 2000 Aug;176:105-15. doi: 10.1034/j.1600-065x.2000.00502.x. Immunol Rev. 2000. PMID: 11043771 Review.
Cited by
-
An In Vitro Evaluation of the Molecular Mechanisms of Action of Medical Plants from the Lamiaceae Family as Effective Sources of Active Compounds against Human Cancer Cell Lines.Cancers (Basel). 2020 Oct 13;12(10):2957. doi: 10.3390/cancers12102957. Cancers (Basel). 2020. PMID: 33066157 Free PMC article. Review.
-
Nucleolin acts as the receptor for C1QTNF4 and supports C1QTNF4-mediated innate immunity modulation.J Biol Chem. 2021 Jan-Jun;296:100513. doi: 10.1016/j.jbc.2021.100513. Epub 2021 Mar 4. J Biol Chem. 2021. PMID: 33676896 Free PMC article.
-
The anti-tumor diterpene oridonin is a direct inhibitor of Nucleolin in cancer cells.Sci Rep. 2018 Nov 13;8(1):16735. doi: 10.1038/s41598-018-35088-x. Sci Rep. 2018. PMID: 30425290 Free PMC article.
-
A MACS protocol for purification of untouched germinal center B cells from unimmunized or germinal center-induced mice.STAR Protoc. 2022 May 14;3(2):101388. doi: 10.1016/j.xpro.2022.101388. eCollection 2022 Jun 17. STAR Protoc. 2022. PMID: 35600926 Free PMC article.
-
The Roles of Sclerostin in Immune System and the Applications of Aptamers in Immune-Related Research.Front Immunol. 2021 Feb 25;12:602330. doi: 10.3389/fimmu.2021.602330. eCollection 2021. Front Immunol. 2021. PMID: 33717084 Free PMC article. Review.
References
-
- Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61(4):212–236. - PubMed
-
- Kondo E, Yoshino T. Expression of apoptosis regulators in germinal centers and germinal center-derived B-cell lymphomas: insight into B-cell lymphomagenesis. Pathol Int. 2007;57(7):391–397. - PubMed
-
- Timmer T, de Vries EG, de Jong S. Fas receptor-mediated apoptosis: a clinical application? J Pathol. 2002;196(2):125–134. - PubMed
-
- Lynch DH, Watson ML, Alderson MR, et al. The mouse Fas-ligand gene is mutated in gld mice and is part of a TNF family gene cluster. Immunity. 1994;1(2):131–136. - PubMed
-
- Rieux-Laucat F, Le Deist F, Hivroz C, Roberts IA, Debatin KM, Fischer A, de Villartay JP. Mutations in Fas associated with human lymphoproliferative syndrome and autoimmunity. Science. 1995;268(5215):1347–1349. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

