Targeting agr- and agr-Like quorum sensing systems for development of common therapeutics to treat multiple gram-positive bacterial infections
- PMID: 23598501
- PMCID: PMC3673130
- DOI: 10.3390/s130405130
Targeting agr- and agr-Like quorum sensing systems for development of common therapeutics to treat multiple gram-positive bacterial infections
Abstract
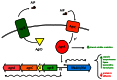
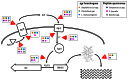
Invasive infection by the Gram-positive pathogen Staphylococcus aureus is controlled by a four gene operon, agr that encodes a quorum sensing system for the regulation of virulence. While agr has been well studied in S. aureus, the contribution of agr homologues and analogues in other Gram-positive pathogens is just beginning to be understood. Intriguingly, other significant human pathogens, including Clostridium perfringens, Listeria monocytogenes, and Enterococcus faecalis contain agr or analogues linked to virulence. Moreover, other significant human Gram-positive pathogens use peptide based quorum sensing systems to establish or maintain infection. The potential for commonality in aspects of these signaling systems across different species raises the prospect of identifying therapeutics that could target multiple pathogens. Here, we review the status of research into these agr homologues, analogues, and other peptide based quorum sensing systems in Gram-positive pathogens as well as the potential for identifying common pathways and signaling mechanisms for therapeutic discovery.
Figures
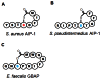
Similar articles
-
Signal Biosynthesis Inhibition with Ambuic Acid as a Strategy To Target Antibiotic-Resistant Infections.Antimicrob Agents Chemother. 2017 Jul 25;61(8):e00263-17. doi: 10.1128/AAC.00263-17. Print 2017 Aug. Antimicrob Agents Chemother. 2017. PMID: 28607020 Free PMC article.
-
Cyclodepsipeptides produced by actinomycetes inhibit cyclic-peptide-mediated quorum sensing in Gram-positive bacteria.FEMS Microbiol Lett. 2015 Jul;362(14):fnv109. doi: 10.1093/femsle/fnv109. Epub 2015 Jul 5. FEMS Microbiol Lett. 2015. PMID: 26149266
-
Alternative approaches to treat bacterial infections: targeting quorum-sensing.Expert Rev Anti Infect Ther. 2020 Jun;18(6):499-510. doi: 10.1080/14787210.2020.1750951. Epub 2020 Apr 13. Expert Rev Anti Infect Ther. 2020. PMID: 32243194 Free PMC article. Review.
-
High-throughput screening of inhibitors targeting Agr/Fsr quorum sensing in Staphylococcus aureus and Enterococcus faecalis.Biosci Biotechnol Biochem. 2013;77(5):923-7. doi: 10.1271/bbb.120769. Epub 2013 May 7. Biosci Biotechnol Biochem. 2013. PMID: 23649251
-
Molecular Mechanism of Quorum-Sensing in Enterococcus faecalis: Its Role in Virulence and Therapeutic Approaches.Int J Mol Sci. 2017 May 3;18(5):960. doi: 10.3390/ijms18050960. Int J Mol Sci. 2017. PMID: 28467378 Free PMC article. Review.
Cited by
-
Identification of the agr Peptide of Listeria monocytogenes.Front Microbiol. 2016 Jun 22;7:989. doi: 10.3389/fmicb.2016.00989. eCollection 2016. Front Microbiol. 2016. PMID: 27446029 Free PMC article.
-
Characterization of MroQ-Dependent Maturation and Export of the Staphylococcus aureus Accessory Gene Regulatory System Autoinducing Peptide.Infect Immun. 2022 Oct 20;90(10):e0026322. doi: 10.1128/iai.00263-22. Epub 2022 Sep 8. Infect Immun. 2022. PMID: 36073934 Free PMC article.
-
Host cell-induced signaling causes Clostridium perfringens to upregulate production of toxins important for intestinal infections.Gut Microbes. 2014 Jan-Feb;5(1):96-107. doi: 10.4161/gmic.26419. Epub 2013 Sep 10. Gut Microbes. 2014. PMID: 24061146 Free PMC article. Review.
-
Selective chemical inhibition of agr quorum sensing in Staphylococcus aureus promotes host defense with minimal impact on resistance.PLoS Pathog. 2014 Jun 12;10(6):e1004174. doi: 10.1371/journal.ppat.1004174. eCollection 2014 Jun. PLoS Pathog. 2014. PMID: 24945495 Free PMC article.
-
Links between S-adenosylmethionine and Agr-based quorum sensing for biofilm development in Listeria monocytogenes EGD-e.Microbiologyopen. 2020 May;9(5):e1015. doi: 10.1002/mbo3.1015. Epub 2020 Mar 5. Microbiologyopen. 2020. PMID: 32134563 Free PMC article.
References
-
- Queck S.Y., Jameson-Lee M., Villaruz A.E., Bach T.-H.L., Khan B., Sturdevant D.E., Ricklefs S.M., Li M., Otto M. RNAIII-independent target gene control by the agr quorum-sensing system: insight into the evolution of virulence regulation in staphylococcus aureus. Mol.Cell. 2008;32:150–158. - PMC - PubMed
-
- Shopsin B., Drlica-Wagner A., Mathema B., Adhikari R.P., Kreiswirth B.N., Novick R.P. Prevalence of agr dysfunction among colonizing Staphylococcus aureus strains. J. Infec. Dis. 2008;198:1171–1174. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

