Microtubule-dependent endosomal sorting of clathrin-independent cargo by Hook1
- PMID: 23589492
- PMCID: PMC3628520
- DOI: 10.1083/jcb.201208172
Microtubule-dependent endosomal sorting of clathrin-independent cargo by Hook1
Abstract
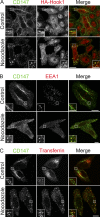
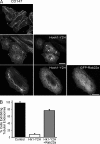
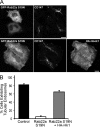
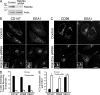
Many plasma membrane (PM) proteins enter cells nonselectively through clathrin-independent endocytosis (CIE). Here, we present evidence that cytoplasmic sequences in three CIE cargo proteins-CD44, CD98, and CD147-were responsible for the rapid sorting of these proteins into endosomal tubules away from endosomes associated with early endosomal antigen 1 (EEA1). We found that Hook1, a microtubule- and cargo-tethering protein, recognized the cytoplasmic tail of CD147 to help sort it and CD98 into Rab22a-dependent tubules associated with recycling. Depletion of Hook1 from cells altered trafficking of CD44, CD98, and CD147 toward EEA1 compartments and impaired the recycling of CD98 back to the PM. In contrast, another CIE cargo protein, major histocompatibility complex class I, which normally traffics to EEA1 compartments, was not affected by depletion of Hook1. Loss of Hook1 also led to an inhibition of cell spreading, implicating a role for Hook1 sorting of specific CIE cargo proteins away from bulk membrane and back to the PM.
Figures
Similar articles
-
Hook1, microtubules, and Rab22: mediators of selective sorting of clathrin-independent endocytic cargo proteins on endosomes.Bioarchitecture. 2013 Sep-Dec;3(5):141-6. doi: 10.4161/bioa.26638. Epub 2013 Sep 1. Bioarchitecture. 2013. PMID: 24284901 Free PMC article.
-
MARCH ubiquitin ligases alter the itinerary of clathrin-independent cargo from recycling to degradation.Mol Biol Cell. 2011 Sep;22(17):3218-30. doi: 10.1091/mbc.E10-11-0874. Epub 2011 Jul 14. Mol Biol Cell. 2011. PMID: 21757542 Free PMC article.
-
Regulation of Hook1-mediated endosomal sorting of clathrin-independent cargo by γ-taxilin.J Cell Sci. 2022 Dec 1;135(1):jcs258849. doi: 10.1242/jcs.258849. Epub 2022 Jan 10. J Cell Sci. 2022. PMID: 34897470
-
Revising Endosomal Trafficking under Insulin Receptor Activation.Int J Mol Sci. 2021 Jun 29;22(13):6978. doi: 10.3390/ijms22136978. Int J Mol Sci. 2021. PMID: 34209489 Free PMC article. Review.
-
Crucial roles of Rab22a in endosomal cargo recycling.Traffic. 2023 Sep;24(9):397-412. doi: 10.1111/tra.12907. Epub 2023 Jun 21. Traffic. 2023. PMID: 37340959 Review.
Cited by
-
Rab and Arf G proteins in endosomal trafficking and cell surface homeostasis.Small GTPases. 2016 Oct;7(4):247-251. doi: 10.1080/21541248.2016.1212687. Epub 2016 Jul 14. Small GTPases. 2016. PMID: 27416526 Free PMC article.
-
HookA is a novel dynein-early endosome linker critical for cargo movement in vivo.J Cell Biol. 2014 Mar 17;204(6):1009-26. doi: 10.1083/jcb.201308009. J Cell Biol. 2014. PMID: 24637327 Free PMC article.
-
Endosomes derived from clathrin-independent endocytosis serve as precursors for endothelial lumen formation.PLoS One. 2013 Nov 25;8(11):e81987. doi: 10.1371/journal.pone.0081987. eCollection 2013. PLoS One. 2013. PMID: 24282620 Free PMC article.
-
A septin GTPase scaffold of dynein-dynactin motors triggers retrograde lysosome transport.J Cell Biol. 2021 Feb 1;220(2):e202005219. doi: 10.1083/jcb.202005219. J Cell Biol. 2021. PMID: 33416861 Free PMC article.
-
HkRP3 is a microtubule-binding protein regulating lytic granule clustering and NK cell killing.J Immunol. 2015 Apr 15;194(8):3984-96. doi: 10.4049/jimmunol.1402897. Epub 2015 Mar 11. J Immunol. 2015. PMID: 25762780 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

