Regulation of matrix assembly through rigidity-dependent fibronectin conformational changes
- PMID: 23589296
- PMCID: PMC3663504
- DOI: 10.1074/jbc.M112.435271
Regulation of matrix assembly through rigidity-dependent fibronectin conformational changes
Abstract
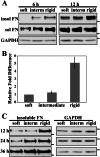
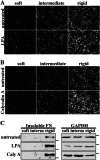
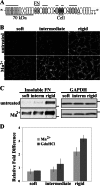
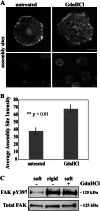
Cells sense and respond to the mechanical properties of their microenvironment. We investigated whether these properties affect the ability of cells to assemble a fibrillar fibronectin (FN) matrix. Analysis of matrix assembled by cells grown on FN-coated polyacrylamide gels of varying stiffnesses showed that rigid substrates stimulate FN matrix assembly and activation of focal adhesion kinase (FAK) compared with the level of assembly and FAK signaling on softer substrates. Stimulating integrins with Mn(2+) treatment increased FN assembly on softer gels, suggesting that integrin binding is deficient on soft substrates. Guanidine hydrochloride-induced extension of the substrate-bound FN rescued assembly on soft substrates to a degree similar to that of Mn(2+) treatment and increased activation of FAK along with the initiation of assembly at FN matrix assembly sites. In contrast, increasing actin-mediated cell contractility did not rescue FN matrix assembly on soft substrates. Thus, rigidity-dependent FN matrix assembly is determined by extracellular events, namely the engagement of FN by cells and the induction of FN conformational changes. Extensibility of FN in response to substrate stiffness may serve as a mechanosensing mechanism whereby cells use pericellular FN to probe the stiffness of their environment.
Keywords: Extracellular Matrix; Fibroblast; Fibronectin; Integrin; Mechanotransduction; Protein Assembly.
Figures


Similar articles
-
The role of integrin binding sites in fibronectin matrix assembly in vivo.Curr Opin Cell Biol. 2008 Oct;20(5):502-7. doi: 10.1016/j.ceb.2008.06.001. Epub 2008 Jul 21. Curr Opin Cell Biol. 2008. PMID: 18586094 Review.
-
Roles of integrins in fibronectin matrix assembly.Histol Histopathol. 1997 Jan;12(1):233-40. Histol Histopathol. 1997. PMID: 9046058 Review.
-
Type III and V collagens modulate the expression and assembly of EDA(+) fibronectin in the extracellular matrix of defective Ehlers-Danlos syndrome fibroblasts.Biochim Biophys Acta. 2012 Oct;1820(10):1576-87. doi: 10.1016/j.bbagen.2012.06.004. Epub 2012 Jun 15. Biochim Biophys Acta. 2012. PMID: 22705941
-
Assembly of fibronectin extracellular matrix.Annu Rev Cell Dev Biol. 2010;26:397-419. doi: 10.1146/annurev-cellbio-100109-104020. Annu Rev Cell Dev Biol. 2010. PMID: 20690820 Free PMC article. Review.
-
The ins and outs of fibronectin matrix assembly.J Cell Sci. 2003 Aug 15;116(Pt 16):3269-76. doi: 10.1242/jcs.00670. J Cell Sci. 2003. PMID: 12857786 Review.
Cited by
-
Matrix compliance and the regulation of cytokinesis.Biol Open. 2015 May 22;4(7):885-92. doi: 10.1242/bio.011825. Biol Open. 2015. PMID: 26002930 Free PMC article.
-
The Soft- and Hard-Heartedness of Cardiac Fibroblasts: Mechanotransduction Signaling Pathways in Fibrosis of the Heart.J Clin Med. 2017 May 19;6(5):53. doi: 10.3390/jcm6050053. J Clin Med. 2017. PMID: 28534817 Free PMC article. Review.
-
ILK supports RhoA/ROCK-mediated contractility of human intestinal epithelial crypt cells by inducing the fibrillogenesis of endogenous soluble fibronectin during the spreading process.BMC Mol Cell Biol. 2020 Mar 17;21(1):14. doi: 10.1186/s12860-020-00259-0. BMC Mol Cell Biol. 2020. PMID: 32183701 Free PMC article.
-
Matrix stiffness controls megakaryocyte adhesion, fibronectin fibrillogenesis, and proplatelet formation through Itgβ3.Blood Adv. 2023 Aug 8;7(15):4003-4018. doi: 10.1182/bloodadvances.2022008680. Blood Adv. 2023. PMID: 37171626 Free PMC article.
-
Conformational coupling of integrin and Thy-1 regulates Fyn priming and fibroblast mechanotransduction.J Cell Biol. 2015 Oct 12;211(1):173-90. doi: 10.1083/jcb.201505007. J Cell Biol. 2015. PMID: 26459603 Free PMC article.
References
-
- Hynes R. O., Yamada K. M. (eds) (2011) Extracellular Matrix Biology, pp. 1–16, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
-
- Wang H. B., Dembo M., Wang Y. L. (2000) Substrate flexibility regulates growth and apoptosis of normal but not transformed cells. Am. J. Physiol. Cell Physiol. 279, C1345–C1350 - PubMed
-
- Yeung T., Georges P. C., Flanagan L. A., Marg B., Ortiz M., Funaki M., Zahir N., Ming W., Weaver V., Janmey P. A. (2005) Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil. Cytoskeleton 60, 24–34 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

