Genomic editing of the HIV-1 coreceptor CCR5 in adult hematopoietic stem and progenitor cells using zinc finger nucleases
- PMID: 23587921
- PMCID: PMC3677314
- DOI: 10.1038/mt.2013.65
Genomic editing of the HIV-1 coreceptor CCR5 in adult hematopoietic stem and progenitor cells using zinc finger nucleases
Abstract
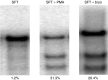
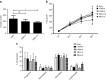
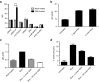
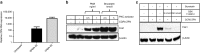
The HIV-1 coreceptor CCR5 is a validated target for HIV/AIDS therapy. The apparent elimination of HIV-1 in a patient treated with an allogeneic stem cell transplant homozygous for a naturally occurring CCR5 deletion mutation (CCR5(Δ32/Δ32)) supports the concept that a single dose of HIV-resistant hematopoietic stem cells can provide disease protection. Given the low frequency of naturally occurring CCR5(Δ32/Δ32) donors, we reasoned that engineered autologous CD34(+) hematopoietic stem/progenitor cells (HSPCs) could be used for AIDS therapy. We evaluated disruption of CCR5 gene expression in HSPCs isolated from granulocyte colony-stimulating factor (CSF)-mobilized adult blood using a recombinant adenoviral vector encoding a CCR5-specific pair of zinc finger nucleases (CCR5-ZFN). Our results demonstrate that CCR5-ZFN RNA and protein expression from the adenoviral vector is enhanced by pretreatment of HSPC with protein kinase C (PKC) activators resulting in >25% CCR5 gene disruption and that activation of the mitogen-activated protein kinase kinase (MEK)/extracellular signal-regulated kinase (ERK) signaling pathway is responsible for this activity. Importantly, using an optimized dose of PKC activator and adenoviral vector we could generate CCR5-modified HSPCs which engraft in a humanized mouse model (albeit at a reduced level) and support multilineage differentiation in vitro and in vivo. Together, these data establish the basis for improved approaches exploiting adenoviral vector delivery in the modification of HSPCs.
Figures
Similar articles
-
Human hematopoietic stem/progenitor cells modified by zinc-finger nucleases targeted to CCR5 control HIV-1 in vivo.Nat Biotechnol. 2010 Aug;28(8):839-47. doi: 10.1038/nbt.1663. Epub 2010 Jul 2. Nat Biotechnol. 2010. PMID: 20601939 Free PMC article.
-
CRISPR/Cas9-Mediated CCR5 Ablation in Human Hematopoietic Stem/Progenitor Cells Confers HIV-1 Resistance In Vivo.Mol Ther. 2017 Aug 2;25(8):1782-1789. doi: 10.1016/j.ymthe.2017.04.027. Epub 2017 May 17. Mol Ther. 2017. PMID: 28527722 Free PMC article.
-
Long-term multilineage engraftment of autologous genome-edited hematopoietic stem cells in nonhuman primates.Blood. 2016 May 19;127(20):2416-26. doi: 10.1182/blood-2015-09-672337. Epub 2016 Mar 15. Blood. 2016. PMID: 26980728 Free PMC article.
-
Editing CCR5: a novel approach to HIV gene therapy.Adv Exp Med Biol. 2015;848:117-30. doi: 10.1007/978-1-4939-2432-5_6. Adv Exp Med Biol. 2015. PMID: 25757618 Review.
-
Pre-clinical modeling of CCR5 knockout in human hematopoietic stem cells by zinc finger nucleases using humanized mice.J Infect Dis. 2013 Nov;208 Suppl 2(Suppl 2):S160-4. doi: 10.1093/infdis/jit382. J Infect Dis. 2013. PMID: 24151324 Free PMC article. Review.
Cited by
-
Development of gene therapy for blood disorders: an update.Blood. 2013 Aug 29;122(9):1556-64. doi: 10.1182/blood-2013-04-453209. Epub 2013 Jul 10. Blood. 2013. PMID: 23843498 Free PMC article. Review.
-
High-Efficiency Transduction of Primary Human Hematopoietic Stem/Progenitor Cells by AAV6 Vectors: Strategies for Overcoming Donor-Variation and Implications in Genome Editing.Sci Rep. 2016 Oct 19;6:35495. doi: 10.1038/srep35495. Sci Rep. 2016. PMID: 27759036 Free PMC article.
-
Efficient genome editing in hematopoietic stem cells with helper-dependent Ad5/35 vectors expressing site-specific endonucleases under microRNA regulation.Mol Ther Methods Clin Dev. 2015 Jan 14;1:14057. doi: 10.1038/mtm.2014.57. eCollection 2015. Mol Ther Methods Clin Dev. 2015. PMID: 26052525 Free PMC article.
-
Gene Editing of Human Hematopoietic Stem and Progenitor Cells: Promise and Potential Hurdles.Hum Gene Ther. 2016 Oct;27(10):729-740. doi: 10.1089/hum.2016.107. Epub 2016 Aug 2. Hum Gene Ther. 2016. PMID: 27483988 Free PMC article. Review.
-
Hematopoietic stem and progenitors cells gene editing: Beyond blood disorders.Front Genome Ed. 2023 Jan 9;4:997142. doi: 10.3389/fgeed.2022.997142. eCollection 2022. Front Genome Ed. 2023. PMID: 36698790 Free PMC article. Review.
References
-
- Hütter G, Nowak D, Mossner M, Ganepola S, Müssig A, Allers K, et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med. 2009;360:692–698. - PubMed
-
- Allers K, Hütter G, Hofmann J, Loddenkemper C, Rieger K, Thiel E, et al. Evidence for the cure of HIV infection by CCR5?32/?32 stem cell transplantation. Blood. 2011;117:2791–2799. - PubMed
-
- Baltimore D. Gene therapy. Intracellular immunization. Nature. 1988;335:395–396. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

