Mapping the crossroads of immune activation and cellular stress response pathways
- PMID: 23584529
- PMCID: PMC3642686
- DOI: 10.1038/emboj.2013.80
Mapping the crossroads of immune activation and cellular stress response pathways
Abstract
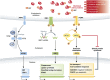
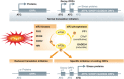
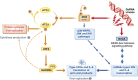
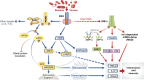
The innate immune cell network detects specific microbes and damages to cell integrity in order to coordinate and polarize the immune response against invading pathogens. In recent years, a cross-talk between microbial-sensing pathways and endoplasmic reticulum (ER) homeostasis has been discovered and have attracted the attention of many researchers from the inflammation field. Abnormal accumulation of proteins in the ER can be seen as a sign of cellular malfunction and triggers a collection of conserved emergency rescue pathways. These signalling cascades, which increase ER homeostasis and favour cell survival, are collectively known as the unfolded protein response (UPR). The induction or activation by microbial stimuli of several molecules linked to the ER stress response pathway have led to the conclusion that microbe sensing by immunocytes is generally associated with an UPR, which serves as a signal amplification cascade favouring inflammatory cytokines production. Induction of the UPR alone was shown to promote inflammation in different cellular and pathological models. Here we discuss how the innate immune and ER-signalling pathways intersect. Moreover, we propose that the induction of UPR-related molecules by microbial products does not necessarily reflect ER stress, but instead is an integral part of a specific transcription programme controlled by innate immunity receptors.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Similar articles
-
At the crossway of ER-stress and proinflammatory responses.FEBS J. 2019 Jan;286(2):297-310. doi: 10.1111/febs.14391. Epub 2018 Feb 6. FEBS J. 2019. PMID: 29360216 Review.
-
Attenuation of PKR-like ER Kinase (PERK) Signaling Selectively Controls Endoplasmic Reticulum Stress-induced Inflammation Without Compromising Immunological Responses.J Biol Chem. 2016 Jul 22;291(30):15830-40. doi: 10.1074/jbc.M116.738021. Epub 2016 May 23. J Biol Chem. 2016. PMID: 27226638 Free PMC article.
-
The endoplasmic reticulum: a sensor of cellular stress that modulates immune responses.Microbes Infect. 2012 Nov;14(14):1293-300. doi: 10.1016/j.micinf.2012.07.005. Epub 2012 Jul 16. Microbes Infect. 2012. PMID: 22800981 Free PMC article. Review.
-
Endoplasmic reticulum stress and unfolded protein response in infection by intracellular parasites.Future Sci OA. 2017 May 12;3(3):FSO198. doi: 10.4155/fsoa-2017-0020. eCollection 2017 Aug. Future Sci OA. 2017. PMID: 28883998 Free PMC article. Review.
-
A Systems Biological View of Life-and-Death Decision with Respect to Endoplasmic Reticulum Stress-The Role of PERK Pathway.Int J Mol Sci. 2017 Jan 5;18(1):58. doi: 10.3390/ijms18010058. Int J Mol Sci. 2017. PMID: 28067773 Free PMC article.
Cited by
-
Innate immunity at mucosal surfaces: the IRE1-RIDD-RIG-I pathway.Trends Immunol. 2015 Jul;36(7):401-9. doi: 10.1016/j.it.2015.05.006. Epub 2015 Jun 17. Trends Immunol. 2015. PMID: 26093676 Free PMC article. Review.
-
Pharmaceutical integrated stress response enhancement protects oligodendrocytes and provides a potential multiple sclerosis therapeutic.Nat Commun. 2015 Mar 13;6:6532. doi: 10.1038/ncomms7532. Nat Commun. 2015. PMID: 25766071 Free PMC article.
-
Green fluorescent protein-based monitoring of endoplasmic reticulum redox poise.Front Genet. 2013 Jun 13;4:108. doi: 10.3389/fgene.2013.00108. eCollection 2013. Front Genet. 2013. PMID: 23781233 Free PMC article.
-
Glucagon-like peptide-1 receptor regulates endoplasmic reticulum stress-induced apoptosis and the associated inflammatory response in chondrocytes and the progression of osteoarthritis in rat.Cell Death Dis. 2018 Feb 12;9(2):212. doi: 10.1038/s41419-017-0217-y. Cell Death Dis. 2018. PMID: 29434185 Free PMC article.
-
Preclinical and Clinical Demonstration of Immunogenicity by mRNA Vaccines against H10N8 and H7N9 Influenza Viruses.Mol Ther. 2017 Jun 7;25(6):1316-1327. doi: 10.1016/j.ymthe.2017.03.035. Epub 2017 Apr 27. Mol Ther. 2017. PMID: 28457665 Free PMC article.
References
-
- Abraham N, Stojdl DF, Duncan PI, Methot N, Ishii T, Dube M, Vanderhyden BC, Atkins HL, Gray DA, McBurney MW, Koromilas AE, Brown EG, Sonenberg N, Bell JC (1999) Characterization of transgenic mice with targeted disruption of the catalytic domain of the double-stranded RNA-dependent protein kinase, PKR. J Biol Chem 274: 5953–5962 - PubMed
-
- Alexopoulou L, Holt AC, Medzhitov R, Flavell RA (2001) Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413: 732–738 - PubMed
-
- Balachandran S, Roberts PC, Brown LE, Truong H, Pattnaik AK, Archer DR, Barber GN (2000) Essential role for the dsRNA-dependent protein kinase PKR in innate immunity to viral infection. Immunity 13: 129–141 - PubMed
-
- Berlanga JJ, Santoyo J, De Haro C (1999) Characterization of a mammalian homolog of the GCN2 eukaryotic initiation factor 2alpha kinase. Eur J Biochem 265: 754–762 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

