The fractalkine/CX3CR1 system regulates β cell function and insulin secretion
- PMID: 23582329
- PMCID: PMC3717389
- DOI: 10.1016/j.cell.2013.03.001
The fractalkine/CX3CR1 system regulates β cell function and insulin secretion
Abstract
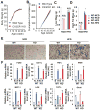
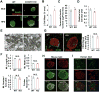
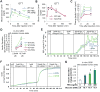
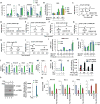
Here, we demonstrate that the fractalkine (FKN)/CX3CR1 system represents a regulatory mechanism for pancreatic islet β cell function and insulin secretion. CX3CR1 knockout (KO) mice exhibited a marked defect in glucose and GLP1-stimulated insulin secretion, and this defect was also observed in vitro in isolated islets from CX3CR1 KO mice. In vivo administration of FKN improved glucose tolerance with an increase in insulin secretion. In vitro treatment of islets with FKN increased intracellular Ca(2+) and potentiated insulin secretion in both mouse and human islets. The KO islets exhibited reduced expression of a set of genes necessary for the fully functional, differentiated β cell state, whereas treatment of wild-type (WT) islets with FKN led to increased expression of these genes. Lastly, expression of FKN in islets was decreased by aging and high-fat diet/obesity, suggesting that decreased FKN/CX3CR1 signaling could be a mechanism underlying β cell dysfunction in type 2 diabetes.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures



Comment in
-
Fractalkine signaling in regulation of insulin secretion.Islets. 2014;6(1):e27861. doi: 10.4161/isl.27861. Islets. 2014. PMID: 25483879 Free PMC article.
Similar articles
-
Fractalkine and its receptor mediate extracellular matrix accumulation in diabetic nephropathy in mice.Diabetologia. 2013 Jul;56(7):1661-9. doi: 10.1007/s00125-013-2907-z. Epub 2013 Apr 19. Diabetologia. 2013. PMID: 23604552 Free PMC article.
-
Fractalkine signaling in regulation of insulin secretion.Islets. 2014;6(1):e27861. doi: 10.4161/isl.27861. Islets. 2014. PMID: 25483879 Free PMC article.
-
Metallothionein 1 negatively regulates glucose-stimulated insulin secretion and is differentially expressed in conditions of beta cell compensation and failure in mice and humans.Diabetologia. 2019 Dec;62(12):2273-2286. doi: 10.1007/s00125-019-05008-3. Epub 2019 Oct 17. Diabetologia. 2019. PMID: 31624901
-
Fractalkine/CX3CR1 axis modulated the development of pancreatic ductal adenocarcinoma via JAK/STAT signaling pathway.Biochem Biophys Res Commun. 2017 Dec 2;493(4):1510-1517. doi: 10.1016/j.bbrc.2017.10.006. Epub 2017 Oct 3. Biochem Biophys Res Commun. 2017. PMID: 28986258
-
Fractalkine/CX3CR1 signalling in chronic pain and inflammation.Curr Pharm Biotechnol. 2011 Oct;12(10):1707-14. doi: 10.2174/138920111798357465. Curr Pharm Biotechnol. 2011. PMID: 21466443 Review.
Cited by
-
Critical Role for Macrophages in the Developmental Programming of Pancreatic β-Cell Area in Offspring of Hypertensive Pregnancies.Diabetes. 2022 Dec 1;71(12):2597-2611. doi: 10.2337/db22-0404. Diabetes. 2022. PMID: 36125850 Free PMC article.
-
Metabolic Impact of MKP-2 Upregulation in Obesity Promotes Insulin Resistance and Fatty Liver Disease.Nutrients. 2022 Jun 15;14(12):2475. doi: 10.3390/nu14122475. Nutrients. 2022. PMID: 35745205 Free PMC article.
-
The Chemokine Systems at the Crossroads of Inflammation and Energy Metabolism in the Development of Obesity.Int J Mol Sci. 2021 Dec 16;22(24):13528. doi: 10.3390/ijms222413528. Int J Mol Sci. 2021. PMID: 34948325 Free PMC article. Review.
-
Chemokine CX3CL1 (Fractalkine) Signaling and Diabetic Encephalopathy.Int J Mol Sci. 2024 Jul 9;25(14):7527. doi: 10.3390/ijms25147527. Int J Mol Sci. 2024. PMID: 39062768 Free PMC article. Review.
-
Nutrient regulation of the islet epigenome controls adaptive insulin secretion.J Clin Invest. 2023 Apr 17;133(8):e165208. doi: 10.1172/JCI165208. J Clin Invest. 2023. PMID: 36821378 Free PMC article.
References
-
- Boehme SA, Lio FM, Maciejewski-Lenoir D, Bacon KB, Conlon PJ. The chemokine fractalkine inhibits Fas-mediated cell death of brain microglia. J Immunol. 2000;165:397–403. - PubMed
-
- Calabrese A, Zhang M, Serre-Beinier V, Caton D, Mas C, Satin LS, Meda P. Connexin 36 controls synchronization of Ca2+ oscillations and insulin secretion in MIN6 cells. Diabetes. 2003;52:417–424. - PubMed
-
- Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, et al. Control of microglial neurotoxicity by the fractalkine receptor. Nature neuroscience. 2006;9:917–924. - PubMed
-
- Carvalho CP, Barbosa HC, Britan A, Santos-Silva JC, Boschero AC, Meda P, Collares-Buzato CB. Beta cell coupling and connexin expression change during the functional maturation of rat pancreatic islets. Diabetologia. 2010;53:1428–1437. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK-033651/DK/NIDDK NIH HHS/United States
- P30 DK063491/DK/NIDDK NIH HHS/United States
- DK-063491/DK/NIDDK NIH HHS/United States
- R01 DK101395/DK/NIDDK NIH HHS/United States
- P30 DK017047/DK/NIDDK NIH HHS/United States
- P01 DK074868/DK/NIDDK NIH HHS/United States
- T32-DK-007494/DK/NIDDK NIH HHS/United States
- R01 DK033651/DK/NIDDK NIH HHS/United States
- U54-HD-012303-25/HD/NICHD NIH HHS/United States
- DK-074868/DK/NIDDK NIH HHS/United States
- T32 DK007494/DK/NIDDK NIH HHS/United States
- U54 HD012303/HD/NICHD NIH HHS/United States
- R37 DK033651/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

