Human mesenchymal stem cells suppress donor CD4(+) T cell proliferation and reduce pathology in a humanized mouse model of acute graft-versus-host disease
- PMID: 23574329
- PMCID: PMC3628335
- DOI: 10.1111/cei.12056
Human mesenchymal stem cells suppress donor CD4(+) T cell proliferation and reduce pathology in a humanized mouse model of acute graft-versus-host disease
Abstract
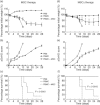
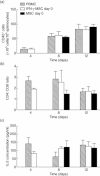
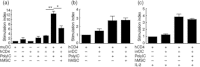
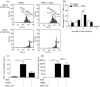
Acute graft-versus-host disease (aGVHD) is a life-threatening complication following allogeneic haematopoietic stem cell transplantation (HSCT), occurring in up to 30-50% of patients who receive human leucocyte antigen (HLA)-matched sibling transplants. Current therapies for steroid refractory aGVHD are limited, with the prognosis of patients suboptimal. Mesenchymal stem or stromal cells (MSC), a heterogeneous cell population present in many tissues, display potent immunomodulatory abilities. Autologous and allogeneic ex-vivo expanded human MSC have been utilized to treat aGVHD with promising results, but the mechanisms of therapeutic action remain unclear. Here a robust humanized mouse model of aGVHD based on delivery of human peripheral blood mononuclear cells (PBMC) to non-obese diabetic (NOD)-severe combined immunodeficient (SCID) interleukin (IL)-2rγ(null) (NSG) mice was developed that allowed the exploration of the role of MSC in cell therapy. MSC therapy resulted in the reduction of liver and gut pathology and significantly increased survival. Protection was dependent upon the timing of MSC therapy, with conventional MSC proving effective only after delayed administration. In contrast, interferon (IFN)-γ-stimulated MSC were effective when delivered with PBMC. The beneficial effect of MSC therapy in this model was not due to the inhibition of donor PBMC chimerism, as CD45(+) and T cells engrafted successfully in this model. MSC therapy did not induce donor T cell anergy, FoxP3(+) T regulatory cells or cause PBMC apoptosis in this model; however, it was associated with the direct inhibition of donor CD4(+) T cell proliferation and reduction of human tumour necrosis factor-α in serum.
© 2012 British Society for Immunology.
Figures




Similar articles
-
Human peripheral blood CD4 T cell-engrafted non-obese diabetic-scid IL2rγ(null) H2-Ab1 (tm1Gru) Tg (human leucocyte antigen D-related 4) mice: a mouse model of human allogeneic graft-versus-host disease.Clin Exp Immunol. 2011 Nov;166(2):269-80. doi: 10.1111/j.1365-2249.2011.04462.x. Clin Exp Immunol. 2011. PMID: 21985373 Free PMC article.
-
The P2X7 receptor antagonist Brilliant Blue G reduces serum human interferon-γ in a humanized mouse model of graft-versus-host disease.Clin Exp Immunol. 2017 Oct;190(1):79-95. doi: 10.1111/cei.13005. Epub 2017 Jul 24. Clin Exp Immunol. 2017. PMID: 28665482 Free PMC article.
-
Extracellular Vesicles Released from Human Umbilical Cord-Derived Mesenchymal Stromal Cells Prevent Life-Threatening Acute Graft-Versus-Host Disease in a Mouse Model of Allogeneic Hematopoietic Stem Cell Transplantation.Stem Cells Dev. 2016 Dec 15;25(24):1874-1883. doi: 10.1089/scd.2016.0107. Epub 2016 Oct 24. Stem Cells Dev. 2016. PMID: 27649744
-
The role of mesenchymal stem cells in hematopoietic stem cell transplantation: prevention and treatment of graft-versus-host disease.Stem Cell Res Ther. 2019 Jun 21;10(1):182. doi: 10.1186/s13287-019-1287-9. Stem Cell Res Ther. 2019. PMID: 31227011 Free PMC article. Review.
-
Chimerism of bone marrow mesenchymal stem/stromal cells in allogeneic hematopoietic cell transplantation: is it clinically relevant?Chimerism. 2013 Jul-Sep;4(3):78-83. doi: 10.4161/chim.25609. Epub 2013 Jul 11. Chimerism. 2013. PMID: 23880502 Free PMC article. Review.
Cited by
-
Hepatocyte Growth Factor Is Required for Mesenchymal Stromal Cell Protection Against Bleomycin-Induced Pulmonary Fibrosis.Stem Cells Transl Med. 2016 Oct;5(10):1307-1318. doi: 10.5966/sctm.2015-0337. Epub 2016 Jul 7. Stem Cells Transl Med. 2016. PMID: 27388243 Free PMC article.
-
Research Progress on Strategies that can Enhance the Therapeutic Benefits of Mesenchymal Stromal Cells in Respiratory Diseases With a Specific Focus on Acute Respiratory Distress Syndrome and Other Inflammatory Lung Diseases.Front Pharmacol. 2021 Apr 19;12:647652. doi: 10.3389/fphar.2021.647652. eCollection 2021. Front Pharmacol. 2021. PMID: 33953680 Free PMC article. Review.
-
Patient risk stratification and tailored clinical management of post-transplant CMV-, EBV-, and BKV-infections by monitoring virus-specific T-cell immunity.EJHaem. 2021 Jun 1;2(3):428-439. doi: 10.1002/jha2.175. eCollection 2021 Aug. EJHaem. 2021. PMID: 35844677 Free PMC article.
-
Improving engraftment and immune reconstitution in umbilical cord blood transplantation.Front Immunol. 2014 Feb 24;5:68. doi: 10.3389/fimmu.2014.00068. eCollection 2014. Front Immunol. 2014. PMID: 24605111 Free PMC article. Review.
-
Interferon-gamma modification of mesenchymal stem cells: implications of autologous and allogeneic mesenchymal stem cell therapy in allotransplantation.Stem Cell Rev Rep. 2014 Jun;10(3):351-75. doi: 10.1007/s12015-014-9495-2. Stem Cell Rev Rep. 2014. PMID: 24510581 Review.
References
-
- Reddy P, Ferrara JL. Immunobiology of acute graft-versus-host disease. Blood Rev. 2003;17:187–194. - PubMed
-
- Billingham RE. The biology of graft-versus-host reactions. Harvey Lect. 1966;62:21–78. - PubMed
-
- Messina C, Faraci M, de Fazio V, Dini G, Calo MP, Calore E. Prevention and treatment of acute GvHD. Bone Marrow Transplant. 2008;41(Suppl. 2):S65–70. - PubMed
-
- Van Lint MT, Uderzo C, Locasciulli A, et al. Early treatment of acute graft-versus-host disease with high- or low-dose 6-methylprednisolone: a multicenter randomized trial from the Italian Group for Bone Marrow Transplantation. Blood. 1998;92:2288–2293. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

