Surface modification of TPGS-b-(PCL-ran-PGA) nanoparticles with polyethyleneimine as a co-delivery system of TRAIL and endostatin for cervical cancer gene therapy
- PMID: 23570619
- PMCID: PMC3639870
- DOI: 10.1186/1556-276X-8-161
Surface modification of TPGS-b-(PCL-ran-PGA) nanoparticles with polyethyleneimine as a co-delivery system of TRAIL and endostatin for cervical cancer gene therapy
Abstract
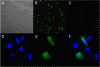
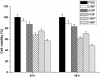
The efficient delivery of therapeutic genes into cells of interest is a critical challenge to broad application of non-viral vector systems. In this research, a novel TPGS-b-(PCL-ran-PGA) nanoparticle modified with polyethyleneimine was applied to be a vector of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and endostatin for cervical cancer gene therapy. Firstly, a novel biodegradable copolymer, TPGS-b-(PCL-ran-PGA), was synthesized and characterized. The nanoparticles were fabricated by an emulsion/solvent evaporation method and then further modified with polyethyleneimine (PEI) carrying TRAIL and/or endostatin genes. The uptake of pIRES2-EGFP and/or pDsRED nanoparticles by HeLa cells were observed by fluorescence microscopy and confocal laser scanning microscopy. The cell viability of TRAIL/endostatin-loaded nanoparticles in HeLa cells was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide assay. Severe combined immunodeficient mice carrying HeLa tumor xenografts were treated in groups of six including phosphate-buffered saline control, blank TPGS-b-(PCL-ran-PGA) nanoparticles, blank TPGS-b-(PCL-ran-PGA)/PEI nanoparticles, and three types of gene nanoparticles. The activity was assessed using average increase in survival time, body weight, and solid tumor volume. All the specimens were then prepared as formalin-fixed and paraffin-embedded tissue sections for hematoxylin-eosin staining. The data showed that the nanoparticles could efficiently deliver plasmids into HeLa cells. The cytotoxicity of the HeLa cells was significantly increased by TRAIL/endostatin-loaded nanoparticles when compared with control groups. The use of TPGS in combination with TRAIL and endostatin had synergistic antitumor effects. In conclusion, the TRAIL/endostatin-loaded nanoparticles offer considerable potential as an ideal candidate for in vivo cancer gene delivery.
Figures




Similar articles
-
Co-delivery of docetaxel and endostatin by a biodegradable nanoparticle for the synergistic treatment of cervical cancer.Nanoscale Res Lett. 2012 Dec 6;7(1):666. doi: 10.1186/1556-276X-7-666. Nanoscale Res Lett. 2012. PMID: 23216701 Free PMC article.
-
Nanoformulation of D-α-tocopheryl polyethylene glycol 1000 succinate-b-poly(ε-caprolactone-ran-glycolide) diblock copolymer for breast cancer therapy.Integr Biol (Camb). 2011 Oct;3(10):993-1002. doi: 10.1039/c1ib00026h. Epub 2011 Sep 22. Integr Biol (Camb). 2011. PMID: 21938302
-
Antitumor efficiency of D-alpha-tocopheryl polyethylene glycol 1000 succinate-b-poly(epsilon-caprolactone-ran-lactide) nanoparticle-based delivery of docetaxel in mice bearing cervical cancer.J Biomed Nanotechnol. 2014 Aug;10(8):1509-19. doi: 10.1166/jbn.2014.1844. J Biomed Nanotechnol. 2014. PMID: 25016651
-
Enhanced anticancer activity of DM1-loaded star-shaped folate-core PLA-TPGS nanoparticles.Nanoscale Res Lett. 2014 Oct 9;9(1):563. doi: 10.1186/1556-276X-9-563. eCollection 2014. Nanoscale Res Lett. 2014. PMID: 25339854 Free PMC article.
-
Antifungal efficacy of itraconazole-loaded TPGS-b-(PCL-ran-PGA) nanoparticles.Int J Nanomedicine. 2015 Feb 17;10:1415-23. doi: 10.2147/IJN.S71616. eCollection 2015. Int J Nanomedicine. 2015. PMID: 25733833 Free PMC article.
Cited by
-
Nano-TRAIL: a promising path to cancer therapy.Cancer Drug Resist. 2023 Feb 1;6(1):78-102. doi: 10.20517/cdr.2022.82. eCollection 2023. Cancer Drug Resist. 2023. PMID: 37065863 Free PMC article. Review.
-
Investigations on agglomeration and haemocompatibility of vitamin E TPGS surface modified berberine chloride nanoparticles.Biomed Res Int. 2014;2014:951942. doi: 10.1155/2014/951942. Epub 2014 Aug 4. Biomed Res Int. 2014. PMID: 25162037 Free PMC article.
-
Recent Advances in the Application of Vitamin E TPGS for Drug Delivery.Theranostics. 2018 Jan 1;8(2):464-485. doi: 10.7150/thno.22711. eCollection 2018. Theranostics. 2018. PMID: 29290821 Free PMC article. Review.
-
Novel Insight of CircRNAs in Cervical Cancer: Potential Biomarkers and Therapeutic Target.Front Med (Lausanne). 2022 Jun 23;9:759928. doi: 10.3389/fmed.2022.759928. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35814779 Free PMC article. Review.
-
Nip the HPV encoded evil in the cancer bud: HPV reshapes TRAILs and signaling landscapes.Cancer Cell Int. 2013 Jun 17;13(1):61. doi: 10.1186/1475-2867-13-61. Cancer Cell Int. 2013. PMID: 23773282 Free PMC article.
References
-
- Ma Y, Huang L, Song C, Zeng X, Liu G, Mei L. Nanoparticle formulation of poly(ε-caprolactone-co-lactide)-d-α-tocopheryl polyethylene glycol 1000 succinate random copolymer for cervical cancer treatment. Polymer. 2010;8:5952–5959. doi: 10.1016/j.polymer.2010.10.029. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

