From neural development to cognition: unexpected roles for chromatin
- PMID: 23568486
- PMCID: PMC4010428
- DOI: 10.1038/nrg3413
From neural development to cognition: unexpected roles for chromatin
Erratum in
- Nat Rev Genet. 2013 Jun;14(6):440
Abstract
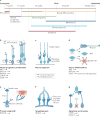
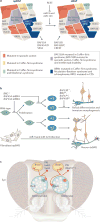
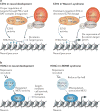
Recent genome-sequencing studies in human neurodevelopmental and psychiatric disorders have uncovered mutations in many chromatin regulators. These human genetic studies, along with studies in model organisms, are providing insight into chromatin regulatory mechanisms in neural development and how alterations to these mechanisms can cause cognitive deficits, such as intellectual disability. We discuss several implicated chromatin regulators, including BAF (also known as SWI/SNF) and CHD8 chromatin remodellers, HDAC4 and the Polycomb component EZH2. Interestingly, mutations in EZH2 and certain BAF complex components have roles in both neurodevelopmental disorders and cancer, and overlapping point mutations are suggesting functionally important residues and domains. We speculate on the contribution of these similar mutations to disparate disorders.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Similar articles
-
Histone deacetylase inhibitors deplete enhancer of zeste 2 and associated polycomb repressive complex 2 proteins in human acute leukemia cells.Mol Cancer Ther. 2006 Dec;5(12):3096-104. doi: 10.1158/1535-7163.MCT-06-0418. Mol Cancer Ther. 2006. PMID: 17172412
-
The role of BAF (mSWI/SNF) complexes in mammalian neural development.Am J Med Genet C Semin Med Genet. 2014 Sep;166C(3):333-49. doi: 10.1002/ajmg.c.31416. Epub 2014 Sep 5. Am J Med Genet C Semin Med Genet. 2014. PMID: 25195934 Free PMC article.
-
PRC2-mediated repression of SMARCA2 predicts EZH2 inhibitor activity in SWI/SNF mutant tumors.Proc Natl Acad Sci U S A. 2017 Nov 14;114(46):12249-12254. doi: 10.1073/pnas.1703966114. Epub 2017 Oct 30. Proc Natl Acad Sci U S A. 2017. PMID: 29087303 Free PMC article.
-
Importance of Ezh2 polycomb protein in tumorigenesis process interfering with the pathway of growth suppressive key elements.J Cell Physiol. 2008 Feb;214(2):295-300. doi: 10.1002/jcp.21241. J Cell Physiol. 2008. PMID: 17786943 Review.
-
PRC2 and SWI/SNF Chromatin Remodeling Complexes in Health and Disease.Biochemistry. 2016 Mar 22;55(11):1600-14. doi: 10.1021/acs.biochem.5b01191. Epub 2016 Feb 17. Biochemistry. 2016. PMID: 26836503 Review.
Cited by
-
The Deficiency of the ASD-Related Gene CHD8 Disrupts Behavioral Patterns and Inhibits Hippocampal Neurogenesis in Mice.J Mol Neurosci. 2024 Oct 31;74(4):103. doi: 10.1007/s12031-024-02283-7. J Mol Neurosci. 2024. PMID: 39480606
-
Hi-C Chromatin Interaction Networks Predict Co-expression in the Mouse Cortex.PLoS Comput Biol. 2015 May 12;11(5):e1004221. doi: 10.1371/journal.pcbi.1004221. eCollection 2015 May. PLoS Comput Biol. 2015. PMID: 25965262 Free PMC article.
-
New Face for Chromatin-Related Mesenchymal Modulator: n-CHD9 Localizes to Nucleoli and Interacts With Ribosomal Genes.J Cell Physiol. 2015 Sep;230(9):2270-80. doi: 10.1002/jcp.24960. J Cell Physiol. 2015. PMID: 25689118 Free PMC article.
-
Neurobiology of ARID1B haploinsufficiency related to neurodevelopmental and psychiatric disorders.Mol Psychiatry. 2022 Jan;27(1):476-489. doi: 10.1038/s41380-021-01060-x. Epub 2021 Mar 8. Mol Psychiatry. 2022. PMID: 33686214 Free PMC article. Review.
-
Sentinels of chromatin: chromodomain helicase DNA-binding proteins in development and disease.Genes Dev. 2021 Nov 1;35(21-22):1403-1430. doi: 10.1101/gad.348897.121. Genes Dev. 2021. PMID: 34725129 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

