Natural variation in Fc glycosylation of HIV-specific antibodies impacts antiviral activity
- PMID: 23563315
- PMCID: PMC3637034
- DOI: 10.1172/JCI65708
Natural variation in Fc glycosylation of HIV-specific antibodies impacts antiviral activity
Abstract
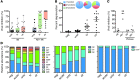
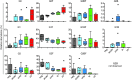
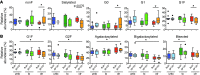
While the induction of a neutralizing antibody response against HIV remains a daunting goal, data from both natural infection and vaccine-induced immune responses suggest that it may be possible to induce antibodies with enhanced Fc effector activity and improved antiviral control via vaccination. However, the specific features of naturally induced HIV-specific antibodies that allow for the potent recruitment of antiviral activity and the means by which these functions are regulated are poorly defined. Because antibody effector functions are critically dependent on antibody Fc domain glycosylation, we aimed to define the natural glycoforms associated with robust Fc-mediated antiviral activity. We demonstrate that spontaneous control of HIV and improved antiviral activity are associated with a dramatic shift in the global antibody-glycosylation profile toward agalactosylated glycoforms. HIV-specific antibodies exhibited an even greater frequency of agalactosylated, afucosylated, and asialylated glycans. These glycoforms were associated with enhanced Fc-mediated reduction of viral replication and enhanced Fc receptor binding and were consistent with transcriptional profiling of glycosyltransferases in peripheral B cells. These data suggest that B cell programs tune antibody glycosylation actively in an antigen-specific manner, potentially contributing to antiviral control during HIV infection.
Figures



Similar articles
-
Antigen-Specific Antibody Glycosylation Is Regulated via Vaccination.PLoS Pathog. 2016 Mar 16;12(3):e1005456. doi: 10.1371/journal.ppat.1005456. eCollection 2016 Mar. PLoS Pathog. 2016. PMID: 26982805 Free PMC article.
-
Distinct Immunoglobulin Fc Glycosylation Patterns Are Associated with Disease Nonprogression and Broadly Neutralizing Antibody Responses in Children with HIV Infection.mSphere. 2020 Dec 23;5(6):e00880-20. doi: 10.1128/mSphere.00880-20. mSphere. 2020. PMID: 33361123 Free PMC article.
-
Diverse antiviral IgG effector activities are predicted by unique biophysical antibody features.Retrovirology. 2021 Oct 30;18(1):35. doi: 10.1186/s12977-021-00579-9. Retrovirology. 2021. PMID: 34717659 Free PMC article.
-
Opportunities to exploit non-neutralizing HIV-specific antibody activity.Curr HIV Res. 2013 Jul;11(5):365-77. doi: 10.2174/1570162x113116660058. Curr HIV Res. 2013. PMID: 24191934 Free PMC article. Review.
-
Role of Fc-mediated antibody function in protective immunity against HIV-1.Immunology. 2014 May;142(1):46-57. doi: 10.1111/imm.12232. Immunology. 2014. PMID: 24843871 Free PMC article. Review.
Cited by
-
Infection and the Glycome─New Insights into Host Response.ACS Infect Dis. 2024 Aug 9;10(8):2540-2550. doi: 10.1021/acsinfecdis.4c00315. Epub 2024 Jul 11. ACS Infect Dis. 2024. PMID: 38990078 Free PMC article. Review.
-
Engineering the supernatural: monoclonal antibodies for challenging infectious diseases.Curr Opin Biotechnol. 2022 Dec;78:102818. doi: 10.1016/j.copbio.2022.102818. Epub 2022 Oct 12. Curr Opin Biotechnol. 2022. PMID: 36242952 Free PMC article. Review.
-
Multiple viral proteins and immune response pathways act to generate robust long-term immunity in Sudan virus survivors.EBioMedicine. 2019 Aug;46:215-226. doi: 10.1016/j.ebiom.2019.07.021. Epub 2019 Jul 17. EBioMedicine. 2019. PMID: 31326432 Free PMC article.
-
Response to immune complex vaccine in chronic hepatitis B patients is associated with lower baseline level of serum IgG galactosylation.Medicine (Baltimore). 2019 Jun;98(26):e16208. doi: 10.1097/MD.0000000000016208. Medicine (Baltimore). 2019. PMID: 31261570 Free PMC article. Clinical Trial.
-
Frontline Science: Plasma and immunoglobulin G galactosylation associate with HIV persistence during antiretroviral therapy.J Leukoc Biol. 2018 Sep;104(3):461-471. doi: 10.1002/JLB.3HI1217-500R. Epub 2018 Apr 6. J Leukoc Biol. 2018. PMID: 29633346 Free PMC article.
References
-
- Baum LL, et al. HIV-1 gp120-specific antibody-dependent cell-mediated cytotoxicity correlates with rate of disease progression. J Immunol. 1996;157(5):2168–2173. - PubMed
-
- Asmal M, et al. Antibody-dependent cell-mediated viral inhibition emerges after simian immunodeficiency virus SIVmac251 infection of rhesus monkeys coincident with gp140-binding antibodies and is effective against neutralization-resistant viruses. J Virol. 2011;85(11):5465–5475. doi: 10.1128/JVI.00313-11. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

