Molecular organization and ATP-induced conformational changes of ABCA4, the photoreceptor-specific ABC transporter
- PMID: 23562398
- PMCID: PMC3654078
- DOI: 10.1016/j.str.2013.03.001
Molecular organization and ATP-induced conformational changes of ABCA4, the photoreceptor-specific ABC transporter
Abstract
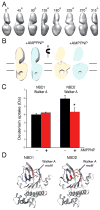
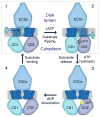
ATP-binding cassette (ABC) transporters use ATP to translocate various substrates across cellular membranes. Several members of subfamily A of mammalian ABC transporters are associated with severe health disorders, but their unusual complexity and large size have so far precluded structural characterization. ABCA4 is localized to the discs of vertebrate photoreceptor outer segments. This protein transports N-retinylidene-phosphatidylethanolamine to the outer side of disc membranes to prevent formation of toxic compounds causing macular degeneration. An 18 Å-resolution structure of ABCA4 isolated from bovine rod outer segments was determined using electron microscopy and single-particle reconstruction. Significant conformational changes in the cytoplasmic and transmembrane regions were observed upon binding of a nonhydrolyzable ATP analog and accompanied by altered hydrogen/deuterium exchange in the Walker A motif of one of the nucleotide-binding domains. These findings provide an initial view of the molecular organization and functional rearrangements for any member of the ABCA subfamily of ABC transporters.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures


Similar articles
-
N-retinylidene-phosphatidylethanolamine is the preferred retinoid substrate for the photoreceptor-specific ABC transporter ABCA4 (ABCR).J Biol Chem. 2004 Dec 24;279(52):53972-9. doi: 10.1074/jbc.M405216200. Epub 2004 Oct 7. J Biol Chem. 2004. PMID: 15471866
-
The ATP-binding cassette transporter ABCA4: structural and functional properties and role in retinal disease.Adv Exp Med Biol. 2010;703:105-25. doi: 10.1007/978-1-4419-5635-4_8. Adv Exp Med Biol. 2010. PMID: 20711710 Free PMC article. Review.
-
ATP-binding cassette transporter ABCA4: molecular properties and role in vision and macular degeneration.J Bioenerg Biomembr. 2007 Dec;39(5-6):507-17. doi: 10.1007/s10863-007-9118-6. J Bioenerg Biomembr. 2007. PMID: 17994272
-
Structure and function of ABCA4 and its role in the visual cycle and Stargardt macular degeneration.Prog Retin Eye Res. 2022 Jul;89:101036. doi: 10.1016/j.preteyeres.2021.101036. Epub 2021 Dec 23. Prog Retin Eye Res. 2022. PMID: 34954332 Review.
-
ATP-binding cassette transporter ABCA4 and chemical isomerization protect photoreceptor cells from the toxic accumulation of excess 11-cis-retinal.Proc Natl Acad Sci U S A. 2014 Apr 1;111(13):5024-9. doi: 10.1073/pnas.1400780111. Epub 2014 Mar 20. Proc Natl Acad Sci U S A. 2014. PMID: 24707049 Free PMC article.
Cited by
-
Expression, purification and structural properties of ABC transporter ABCA4 and its individual domains.Protein Expr Purif. 2014 May;97:50-60. doi: 10.1016/j.pep.2014.02.010. Epub 2014 Feb 28. Protein Expr Purif. 2014. PMID: 24583180 Free PMC article.
-
Molecular components affecting ocular carotenoid and retinoid homeostasis.Prog Retin Eye Res. 2021 Jan;80:100864. doi: 10.1016/j.preteyeres.2020.100864. Epub 2020 Apr 25. Prog Retin Eye Res. 2021. PMID: 32339666 Free PMC article. Review.
-
An Overview of the Genetics of ABCA4 Retinopathies, an Evolving Story.Genes (Basel). 2021 Aug 13;12(8):1241. doi: 10.3390/genes12081241. Genes (Basel). 2021. PMID: 34440414 Free PMC article. Review.
-
Next-Generation Sequencing-Aided Rapid Molecular Diagnosis of Occult Macular Dystrophy in a Chinese Family.Front Genet. 2017 Aug 25;8:107. doi: 10.3389/fgene.2017.00107. eCollection 2017. Front Genet. 2017. PMID: 28890726 Free PMC article.
-
Retinoids and Retinal Diseases.Annu Rev Vis Sci. 2016 Oct;2:197-234. doi: 10.1146/annurev-vision-111815-114407. Epub 2016 Jul 18. Annu Rev Vis Sci. 2016. PMID: 27917399 Free PMC article. Review.
References
-
- Ahn J, Beharry S, Molday LL, Molday RS. Functional interaction between the two halves of the photoreceptor-specific ATP binding cassette protein ABCR (ABCA4). Evidence for a non-exchangeable ADP in the first nucleotide binding domain. J Biol Chem. 2003;278:39600–39608. - PubMed
-
- Ahn J, Wong JT, Molday RS. The effect of lipid environment and retinoids on the ATPase activity of ABCR, the photoreceptor ABC transporter responsible for Stargardt macular dystrophy. J Biol Chem. 2000;275:20399–20405. - PubMed
-
- Aleksandrov L, Aleksandrov AA, Chang XB, Riordan JR. The First Nucleotide Binding Domain of Cystic Fibrosis Transmembrane Conductance Regulator Is a Site of Stable Nucleotide Interaction, whereas the Second Is a Site of Rapid Turnover. J Biol Chem. 2002;277:15419–15425. - PubMed
-
- Allikmets R, Shroyer NF, Singh N, Seddon JM, Lewis RA, Bernstein PS, Peiffer A, Zabriskie NA, Li Y, Hutchinson A, et al. Mutation of the Stargardt disease gene (ABCR) in age-related macular degeneration. Science. 1997a;277:1805–1807. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

