Pandemic influenza A viruses escape from restriction by human MxA through adaptive mutations in the nucleoprotein
- PMID: 23555271
- PMCID: PMC3610643
- DOI: 10.1371/journal.ppat.1003279
Pandemic influenza A viruses escape from restriction by human MxA through adaptive mutations in the nucleoprotein
Abstract
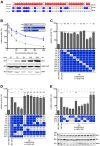
The interferon-induced dynamin-like MxA GTPase restricts the replication of influenza A viruses. We identified adaptive mutations in the nucleoprotein (NP) of pandemic strains A/Brevig Mission/1/1918 (1918) and A/Hamburg/4/2009 (pH1N1) that confer MxA resistance. These resistance-associated amino acids in NP differ between the two strains but form a similar discrete surface-exposed cluster in the body domain of NP, indicating that MxA resistance evolved independently. The 1918 cluster was conserved in all descendent strains of seasonal influenza viruses. Introduction of this cluster into the NP of the MxA-sensitive influenza virus A/Thailand/1(KAN-1)/04 (H5N1) resulted in a gain of MxA resistance coupled with a decrease in viral replication fitness. Conversely, introduction of MxA-sensitive amino acids into pH1N1 NP enhanced viral growth in Mx-negative cells. We conclude that human MxA represents a barrier against zoonotic introduction of avian influenza viruses and that adaptive mutations in the viral NP should be carefully monitored.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
The viral nucleoprotein determines Mx sensitivity of influenza A viruses.J Virol. 2011 Aug;85(16):8133-40. doi: 10.1128/JVI.00712-11. Epub 2011 Jun 15. J Virol. 2011. PMID: 21680506 Free PMC article.
-
Eurasian Avian-Like Swine Influenza A Viruses Escape Human MxA Restriction through Distinct Mutations in Their Nucleoprotein.J Virol. 2019 Jan 4;93(2):e00997-18. doi: 10.1128/JVI.00997-18. J Virol. 2019. PMID: 30355693 Free PMC article.
-
The nucleoprotein of newly emerged H7N9 influenza A virus harbors a unique motif conferring resistance to antiviral human MxA.J Virol. 2015 Feb;89(4):2241-52. doi: 10.1128/JVI.02406-14. Epub 2014 Dec 10. J Virol. 2015. PMID: 25505067 Free PMC article.
-
Mx genes: host determinants controlling influenza virus infection and trans-species transmission.Hum Genet. 2020 Jun;139(6-7):695-705. doi: 10.1007/s00439-019-02092-8. Epub 2019 Nov 26. Hum Genet. 2020. PMID: 31773252 Free PMC article. Review.
-
Mx proteins: GTPases involved in the interferon-induced antiviral state.Ciba Found Symp. 1993;176:233-43; discussion 243-7. doi: 10.1002/9780470514450.ch15. Ciba Found Symp. 1993. PMID: 7507812 Review.
Cited by
-
Two complete 1918 influenza A/H1N1 pandemic virus genomes characterized by next-generation sequencing using RNA isolated from formalin-fixed, paraffin-embedded autopsy lung tissue samples along with evidence of secondary bacterial co-infection.mBio. 2024 Mar 13;15(3):e0321823. doi: 10.1128/mbio.03218-23. Epub 2024 Feb 13. mBio. 2024. PMID: 38349163 Free PMC article.
-
Zoonotic infection with swine A/H1avN1 influenza virus in a child, Germany, June 2020.Euro Surveill. 2020 Oct;25(42):2001638. doi: 10.2807/1560-7917.ES.2020.25.42.2001638. Euro Surveill. 2020. PMID: 33094718 Free PMC article.
-
Mammalian-adaptive mutation NP-Q357K in Eurasian H1N1 Swine Influenza viruses determines the virulence phenotype in mice.Emerg Microbes Infect. 2019;8(1):989-999. doi: 10.1080/22221751.2019.1635873. Emerg Microbes Infect. 2019. PMID: 31267843 Free PMC article.
-
The RNA-dependent RNA polymerase of the influenza A virus.Future Virol. 2014 Sep;9(9):863-876. doi: 10.2217/fvl.14.66. Future Virol. 2014. PMID: 25431616 Free PMC article.
-
Expansion of genotypic diversity and establishment of 2009 H1N1 pandemic-origin internal genes in pigs in China.J Virol. 2014 Sep;88(18):10864-74. doi: 10.1128/JVI.01327-14. Epub 2014 Jul 9. J Virol. 2014. PMID: 25008935 Free PMC article.
References
-
- Malik Peiris JS (2009) Avian influenza viruses in humans. Rev Sci Tech 28: 161–173. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

