Local arginase 1 activity is required for cutaneous wound healing
- PMID: 23552798
- PMCID: PMC3778883
- DOI: 10.1038/jid.2013.164
Local arginase 1 activity is required for cutaneous wound healing
Abstract
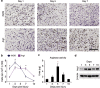
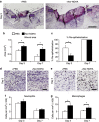
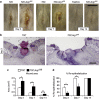
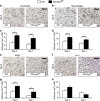
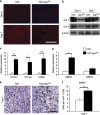
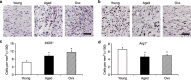
Chronic nonhealing wounds in the elderly population are associated with a prolonged and excessive inflammatory response, which is widely hypothesized to impede healing. Previous studies have linked alterations in local L-arginine metabolism, principally mediated by the enzymes arginase (Arg) and inducible nitric oxide synthase (iNOS), to pathological wound healing. Over subsequent years, interest in Arg/iNOS has focused on the classical versus alternatively activated (M1/M2) macrophage paradigm. Although the role of iNOS during healing has been studied, Arg contribution to healing remains unclear. Here, we report that Arg is dynamically regulated during acute wound healing. Pharmacological inhibition of local Arg activity directly perturbed healing, as did Tie2-cre-mediated deletion of Arg1, revealing the importance of Arg1 during healing. Inhibition or depletion of Arg did not alter alternatively activated macrophage numbers but instead was associated with increased inflammation, including increased influx of iNOS(+) cells and defects in matrix deposition. Finally, we reveal that in preclinical murine models reduced Arg expression directly correlates with delayed healing, and as such may represent an important future therapeutic target.
Figures
Similar articles
-
Expression and activity of arginase isoenzymes during normal and diabetes-impaired skin repair.J Invest Dermatol. 2003 Dec;121(6):1544-51. doi: 10.1046/j.1523-1747.2003.12610.x. J Invest Dermatol. 2003. PMID: 14675208
-
Cellular and physiological upregulation of inducible nitric oxide synthase, arginase, and inducible cyclooxygenase in wound healing.J Cell Physiol. 2019 Dec;234(12):23618-23632. doi: 10.1002/jcp.28930. Epub 2019 Jun 3. J Cell Physiol. 2019. PMID: 31161614
-
Mutual inter-regulation between iNOS and TGF-β1: Possible molecular and cellular mechanisms of iNOS in wound healing.Biochim Biophys Acta Mol Basis Dis. 2020 Oct 1;1866(10):165850. doi: 10.1016/j.bbadis.2020.165850. Epub 2020 Jun 1. Biochim Biophys Acta Mol Basis Dis. 2020. PMID: 32497615
-
Transcriptional regulation of macrophage arginase 1 expression and its role in atherosclerosis.Trends Cardiovasc Med. 2013 Jul;23(5):143-52. doi: 10.1016/j.tcm.2012.10.003. Epub 2013 Jan 30. Trends Cardiovasc Med. 2013. PMID: 23375628 Review.
-
Immune cells in skin inflammation, wound healing, and skin cancer.J Leukoc Biol. 2024 Apr 29;115(5):852-865. doi: 10.1093/jleuko/qiad107. J Leukoc Biol. 2024. PMID: 37718697 Review.
Cited by
-
Topical Wound Treatment with a Nitric Oxide-Releasing PDE5 Inhibitor Formulation Enhances Blood Perfusion and Promotes Healing in Mice.Pharmaceutics. 2022 Oct 31;14(11):2358. doi: 10.3390/pharmaceutics14112358. Pharmaceutics. 2022. PMID: 36365176 Free PMC article.
-
Impaired wound healing in type 1 diabetes is dependent on 5-lipoxygenase products.Sci Rep. 2018 Sep 21;8(1):14164. doi: 10.1038/s41598-018-32589-7. Sci Rep. 2018. PMID: 30242286 Free PMC article.
-
Increased cell proliferation and differential protein expression induced by low-level Er:YAG laser irradiation in human gingival fibroblasts: proteomic analysis.Lasers Med Sci. 2015 Sep;30(7):1855-66. doi: 10.1007/s10103-014-1691-4. Epub 2014 Nov 28. Lasers Med Sci. 2015. PMID: 25429773
-
Adventitial fibroblasts induce a distinct proinflammatory/profibrotic macrophage phenotype in pulmonary hypertension.J Immunol. 2014 Jul 15;193(2):597-609. doi: 10.4049/jimmunol.1303048. Epub 2014 Jun 13. J Immunol. 2014. PMID: 24928992 Free PMC article.
-
Contribution of metabolic reprogramming to macrophage plasticity and function.Semin Immunol. 2015 Aug;27(4):267-75. doi: 10.1016/j.smim.2015.09.001. Epub 2015 Oct 9. Semin Immunol. 2015. PMID: 26454572 Free PMC article. Review.
References
-
- Abd-El-Aleem SA, Ferguson MW, Appleton I, et al. Expression of nitric oxide synthase isoforms and arginase in normal human skin and chronic venous leg ulcers. J Pathol. 2000;191:434–442. - PubMed
-
- Abeyakirthi S, Mowbray M, Bredenkamp N, et al. Arginase is overactive in psoriatic skin. Br J Dermatol. 2010;163:193–196. - PubMed
-
- Albina JE, Mills CD, Henry WL, Jr., et al. Temporal expression of different pathways of 1-arginine metabolism in healing wounds. J Immunol. 1990;144:3877–3880. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

