Ascorbate induces ten-eleven translocation (Tet) methylcytosine dioxygenase-mediated generation of 5-hydroxymethylcytosine
- PMID: 23548903
- PMCID: PMC3650403
- DOI: 10.1074/jbc.C113.464800
Ascorbate induces ten-eleven translocation (Tet) methylcytosine dioxygenase-mediated generation of 5-hydroxymethylcytosine
Abstract
Background: Tet methylcytosine dioxygenase converts 5-mC to 5-hmC in DNA.
Results: Ascorbate significantly and specifically enhances Tet-mediated generation of 5-hmC.
Conclusion: Our findings suggest that ascorbate enhances 5-hmC generation, most likely by acting as a co-factor for Tet methylcytosine dioxygenase to generate 5-hmC.
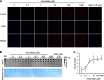
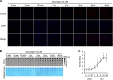
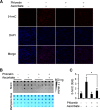
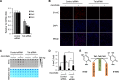
Significance: The availability of ascorbate could have significant consequences for health and diseases by modulating the epigenetic control of genome activity. Ascorbate (vitamin C) is best known for its role in scurvy, in which the hydroxylation of collagen catalyzed by dioxygenases is incomplete due to ascorbate deficiency. Here, we report a novel function of ascorbate in the hydroxylation of 5-methylcytosine (5-mC) to 5-hydroxymethylcytosine (5-hmC) in DNA catalyzed by Tet (ten-eleven translocation) methylcytosine dioxygenase. The content of 5-hmC is extremely low in mouse embryonic fibroblasts cultured in ascorbate-free medium. Additions of ascorbate dose- and time-dependently enhance the generation of 5-hmC, without any effects on the expression of Tet genes. Treatment with another reducer glutathione (GSH) does not change the level of 5-hmC. Further, blocking ascorbate entry into cells by phloretin and knocking down Tet (Tet1, Tet2, and Tet3) expression by short interference RNAs (siRNA) significantly inhibit the effect of ascorbate on 5-hmC. These results suggest that ascorbate enhances 5-hmC generation, most likely by acting as a co-factor for Tet methylcytosine dioxygenase to hydroxylate 5-mC. Thus, we have uncovered a novel role for ascorbate in modulating the epigenetic control of genome activity.
Keywords: 5-Hydroxymethylcytosine; 5-Methylcytosine; DNA Methylation; Enzymes; Epigenetics; Mouse Embryonic Fibroblast; Phloretin; Tet Methylcytosine Dioxygenases; Transport; Vitamin C.
Figures
Similar articles
-
Ascorbate-induced generation of 5-hydroxymethylcytosine is unaffected by varying levels of iron and 2-oxoglutarate.Biochem Biophys Res Commun. 2013 Oct 4;439(4):522-7. doi: 10.1016/j.bbrc.2013.09.010. Epub 2013 Sep 8. Biochem Biophys Res Commun. 2013. PMID: 24021282 Free PMC article.
-
TET family proteins and 5-hydroxymethylcytosine in esophageal squamous cell carcinoma.Oncotarget. 2015 Sep 15;6(27):23372-82. doi: 10.18632/oncotarget.4281. Oncotarget. 2015. PMID: 26093090 Free PMC article.
-
TET-mediated hydroxymethylcytosine at the Pparγ locus is required for initiation of adipogenic differentiation.Int J Obes (Lond). 2017 Apr;41(4):652-659. doi: 10.1038/ijo.2017.8. Epub 2017 Feb 21. Int J Obes (Lond). 2017. PMID: 28100914
-
Epigenetic Function of TET Family, 5-Methylcytosine, and 5-Hydroxymethylcytosine in Hematologic Malignancies.Oncol Res Treat. 2019;42(6):309-318. doi: 10.1159/000498947. Epub 2019 May 3. Oncol Res Treat. 2019. PMID: 31055566 Review.
-
5-hydroxymethylcytosine: a new insight into epigenetics in cancer.Cancer Biol Ther. 2014 Jan;15(1):10-5. doi: 10.4161/cbt.27144. Epub 2013 Nov 19. Cancer Biol Ther. 2014. PMID: 24253310 Free PMC article. Review.
Cited by
-
Epigenetic effects of pharmacologic ascorbate.Oncotarget. 2021 Apr 27;12(9):876-877. doi: 10.18632/oncotarget.27911. eCollection 2021 Apr 27. Oncotarget. 2021. PMID: 33953841 Free PMC article. No abstract available.
-
The TET2 interactors and their links to hematological malignancies.IUBMB Life. 2015 Jun;67(6):438-45. doi: 10.1002/iub.1389. Epub 2015 Jun 22. IUBMB Life. 2015. PMID: 26099018 Free PMC article. Review.
-
The Role of 2-Oxoglutarate Dependent Dioxygenases in Gliomas and Glioblastomas: A Review of Epigenetic Reprogramming and Hypoxic Response.Front Oncol. 2021 Mar 25;11:619300. doi: 10.3389/fonc.2021.619300. eCollection 2021. Front Oncol. 2021. PMID: 33842321 Free PMC article. Review.
-
Iron-dependent epigenetic modulation promotes pathogenic T cell differentiation in lupus.J Clin Invest. 2022 May 2;132(9):e152345. doi: 10.1172/JCI152345. J Clin Invest. 2022. PMID: 35499082 Free PMC article.
-
High Vitamin C Status Is Associated with Elevated Mood in Male Tertiary Students.Antioxidants (Basel). 2018 Jul 16;7(7):91. doi: 10.3390/antiox7070091. Antioxidants (Basel). 2018. PMID: 30012945 Free PMC article.
References
-
- Westbye M. P., Feyzi E., Aas P. A., Vågbø C. B., Talstad V. A., Kavli B., Hagen L., Sundheim O., Akbari M., Liabakk N. B., Slupphaug G., Otterlei M., Krokan H. E. (2008) Human AlkB homolog 1 is a mitochondrial protein that demethylates 3-methylcytosine in DNA and RNA. J. Biol. Chem. 283, 25046–250056 - PMC - PubMed
-
- Zheng G., Dahl J. A., Niu Y., Fedorcsak P., Huang C. M., Li C. J., Vågbø C. B., Shi Y., Wang W. L., Song S. H., Lu Z., Bosmans R. P., Dai Q., Hao Y. J., Yang X., Zhao W. M., Tong W. M., Wang X. J., Bogdan F., Furu K., Fu Y., Jia G., Zhao X., Liu J., Krokan H. E., Klungland A., Yang Y. G., He C. (2013) ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol. Cell 49, 18–29 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

