CD14 protein acts as an adaptor molecule for the immune recognition of Salmonella curli fibers
- PMID: 23548899
- PMCID: PMC3656274
- DOI: 10.1074/jbc.M112.447060
CD14 protein acts as an adaptor molecule for the immune recognition of Salmonella curli fibers
Abstract
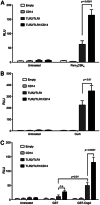
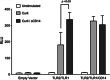
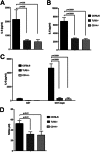
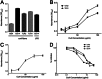
Amyloids, protein aggregates with a cross β-sheet structure, contribute to inflammation in debilitating disorders, including Alzheimer's disease. Enteric bacteria also produce amyloids, termed curli, contributing to inflammation during infection. It has been demonstrated that curli and β-amyloid are recognized by the immune system via the Toll-like receptor (TLR) 2/TLR1 complex. Here we investigated the role of CD14 in the immune recognition of bacterial amyloids. We used HeLa 57A cells, a human cervical cancer cell line containing a luciferase reporter gene under the control of an NF-κB promoter. When HeLa 57A cells were transiently transfected with combinations of human expression vectors containing genes for TLR2, TLR1, and CD14, membrane-bound CD14 enhanced NF-κB activation through the TLR2/TLR1 complex stimulated with curli fibers or recombinant CsgA, the curli major subunit. Similarly, soluble CD14 augmented the TLR2/TLR1 response to curli fibers in the absence of membrane-bound CD14. We further revealed that IL-6 and nitric oxide production were significantly higher by wild-type (C57BL/6) bone marrow-derived macrophages compared with TLR2-deficient or CD14-deficient bone marrow-derived macrophages when stimulated with curli fibers, recombinant CsgA, or synthetic CsgA peptide, CsgA-R4-5. Binding assays demonstrated that recombinant TLR2, TLR1, and CD14 bound purified curli fibers. Interestingly, CD14-curli interaction was specific to the fibrillar form of the amyloid, as demonstrated by using synthetic CsgA peptides proficient and deficient in fiber formation, respectively. Activation of the TLR2/TLR1/CD14 trimolecular complex by amyloids provides novel insights for innate immunity with implications for amyloid-associated diseases.
Keywords: Amyloid; Bacteria; Bacterial Pathogenesis; Biofilm; CD14; Curli; Salmonella; TLR2; Toll-like Receptors (TLR).
Figures

Similar articles
-
Toll-like receptor 2 and NLRP3 cooperate to recognize a functional bacterial amyloid, curli.Infect Immun. 2015 Feb;83(2):693-701. doi: 10.1128/IAI.02370-14. Epub 2014 Nov 24. Infect Immun. 2015. PMID: 25422268 Free PMC article.
-
Bacterial amyloid curli acts as a carrier for DNA to elicit an autoimmune response via TLR2 and TLR9.PLoS Pathog. 2017 Apr 14;13(4):e1006315. doi: 10.1371/journal.ppat.1006315. eCollection 2017 Apr. PLoS Pathog. 2017. PMID: 28410407 Free PMC article.
-
Epithelial cells augment barrier function via activation of the Toll-like receptor 2/phosphatidylinositol 3-kinase pathway upon recognition of Salmonella enterica serovar Typhimurium curli fibrils in the gut.Infect Immun. 2013 Feb;81(2):478-86. doi: 10.1128/IAI.00453-12. Epub 2012 Dec 3. Infect Immun. 2013. PMID: 23208603 Free PMC article.
-
Curli-Containing Enteric Biofilms Inside and Out: Matrix Composition, Immune Recognition, and Disease Implications.Microbiol Mol Biol Rev. 2018 Oct 10;82(4):e00028-18. doi: 10.1128/MMBR.00028-18. Print 2018 Dec. Microbiol Mol Biol Rev. 2018. PMID: 30305312 Free PMC article. Review.
-
Effects of Toll-like receptor 1 and 2 agonist Pam3CSK4 on uveal melanocytes and relevant experimental mouse model.Exp Eye Res. 2024 Feb;239:109749. doi: 10.1016/j.exer.2023.109749. Epub 2023 Dec 17. Exp Eye Res. 2024. PMID: 38113956 Review.
Cited by
-
Fecal microbiota transplantation can improve cognition in patients with cognitive decline and Clostridioides difficile infection.Aging (Albany NY). 2022 Aug 16;14(16):6449-6466. doi: 10.18632/aging.204230. Epub 2022 Aug 16. Aging (Albany NY). 2022. PMID: 35980280 Free PMC article.
-
CD36 Shunts Eicosanoid Metabolism to Repress CD14 Licensed Interleukin-1β Release and Inflammation.Front Immunol. 2018 Apr 27;9:890. doi: 10.3389/fimmu.2018.00890. eCollection 2018. Front Immunol. 2018. PMID: 29755470 Free PMC article.
-
Microbial-generated amyloids and Alzheimer's disease (AD).Front Aging Neurosci. 2015 Feb 10;7:9. doi: 10.3389/fnagi.2015.00009. eCollection 2015. Front Aging Neurosci. 2015. PMID: 25713531 Free PMC article. No abstract available.
-
Community behavior and amyloid-associated phenotypes among a panel of uropathogenic E. coli.Biochem Biophys Res Commun. 2014 Jan 10;443(2):345-50. doi: 10.1016/j.bbrc.2013.11.026. Epub 2013 Nov 15. Biochem Biophys Res Commun. 2014. PMID: 24239885 Free PMC article.
-
Phagocytosis-mediated M1 activation by chitin but not by chitosan.Am J Physiol Cell Physiol. 2018 Jul 1;315(1):C62-C72. doi: 10.1152/ajpcell.00268.2017. Epub 2018 May 2. Am J Physiol Cell Physiol. 2018. PMID: 29719169 Free PMC article.
References
-
- Mok K. H., Pettersson J., Orrenius S., Svanborg C. (2007) HAMLET, protein folding, and tumor cell death. Biochem. Biophys. Res. Commun. 354, 1–7 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

