Mesenchymal stem cells deliver synthetic microRNA mimics to glioma cells and glioma stem cells and inhibit their cell migration and self-renewal
- PMID: 23548312
- PMCID: PMC3712579
- DOI: 10.18632/oncotarget.868
Mesenchymal stem cells deliver synthetic microRNA mimics to glioma cells and glioma stem cells and inhibit their cell migration and self-renewal
Abstract
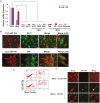
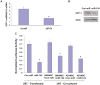
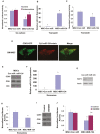
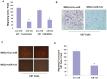
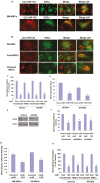
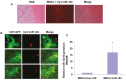
MicroRNAs (miRNAs) have emerged as potential cancer therapeutics; however, their clinical use is hindered by lack of effective delivery mechanisms to tumor sites. Mesenchymal stem cells (MSCs) have been shown to migrate to experimental glioma and to exert anti-tumor effects by delivering cytotoxic compounds. Here, we examined the ability of MSCs derived from bone marrow, adipose tissue, placenta and umbilical cord to deliver synthetic miRNA mimics to glioma cells and glioma stem cells (GSCs). We examined the delivery of miR-124 and miR-145 mimics as glioma cells and GSCs express very low levels of these miRNAs. Using fluorescently labeled miRNA mimics and in situ hybridization, we demonstrated that all the MSCs examined delivered miR-124 and miR-145 mimics to co-cultured glioma cells and GSCs via gap junction- dependent and independent processes. The delivered miR-124 and miR-145 mimics significantly decreased the luciferase activity of their respected reporter target genes, SCP-1 and Sox2, and decreased the migration of glioma cells and the self-renewal of GSCs. Moreover, MSCs delivered Cy3-miR-124 mimic to glioma xenografts when administered intracranially. These results suggest that MSCs can deliver synthetic exogenous miRNA mimics to glioma cells and GSCs and may provide an efficient route of therapeutic miRNA delivery in vivo.
Figures
Similar articles
-
MicroRNA-30a suppresses self-renewal and tumorigenicity of glioma stem cells by blocking the NT5E-dependent Akt signaling pathway.FASEB J. 2020 Apr;34(4):5128-5143. doi: 10.1096/fj.201802629RR. Epub 2020 Feb 17. FASEB J. 2020. PMID: 32067282
-
Mesenchymal stem cell-derived exosomal microRNA-133b suppresses glioma progression via Wnt/β-catenin signaling pathway by targeting EZH2.Stem Cell Res Ther. 2019 Dec 16;10(1):381. doi: 10.1186/s13287-019-1446-z. Stem Cell Res Ther. 2019. PMID: 31842978 Free PMC article.
-
Gene therapy using TRAIL-secreting human umbilical cord blood-derived mesenchymal stem cells against intracranial glioma.Cancer Res. 2008 Dec 1;68(23):9614-23. doi: 10.1158/0008-5472.CAN-08-0451. Cancer Res. 2008. PMID: 19047138
-
New advances of microRNAs in glioma stem cells, with special emphasis on aberrant methylation of microRNAs.J Cell Physiol. 2014 Sep;229(9):1141-7. doi: 10.1002/jcp.24540. J Cell Physiol. 2014. PMID: 24374932 Review.
-
Mesenchymal stem cells as therapeutic vehicles for glioma.Cancer Gene Ther. 2024 Sep;31(9):1306-1314. doi: 10.1038/s41417-024-00775-7. Epub 2024 Apr 23. Cancer Gene Ther. 2024. PMID: 38654128 Review.
Cited by
-
Drug delivery strategies to enhance the permeability of the blood-brain barrier for treatment of glioma.Drug Des Devel Ther. 2015 Apr 9;9:2089-100. doi: 10.2147/DDDT.S79592. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 25926719 Free PMC article. Review.
-
Current Strategies and Therapeutic Applications of Mesenchymal Stem Cell-Based Drug Delivery.Pharmaceuticals (Basel). 2024 May 30;17(6):707. doi: 10.3390/ph17060707. Pharmaceuticals (Basel). 2024. PMID: 38931374 Free PMC article. Review.
-
Effect of bone marrow mesenchymal stem cells on RhoA/ROCK signal pathway in severe acute pancreatitis.Am J Transl Res. 2019 Aug 15;11(8):4809-4816. eCollection 2019. Am J Transl Res. 2019. PMID: 31497201 Free PMC article.
-
Extracellular vesicles: emerging anti-cancer drugs and advanced functionalization platforms for cancer therapy.Drug Deliv. 2022 Dec;29(1):2513-2538. doi: 10.1080/10717544.2022.2104404. Drug Deliv. 2022. PMID: 35915054 Free PMC article. Review.
-
MicroRNAs, Hypoxia and the Stem-Like State as Contributors to Cancer Aggressiveness.Front Genet. 2019 Feb 20;10:125. doi: 10.3389/fgene.2019.00125. eCollection 2019. Front Genet. 2019. PMID: 30842790 Free PMC article. Review.
References
-
- Kleihues P, Louis DN, Scheithauer BW, Rorke LB, Reifenberger G, Burger PC, Cavenee WK. The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol. 2002;61:215–225. - PubMed
-
- Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A, Hahn WC, Ligon KL, Louis DN, Brennan C, Chin L, DePinho RA, Cavenee WK. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–2710. - PubMed
-
- Desjardins A, Rich JN, Quinn JA, Vredenburgh J, Gururangan S, Sathornsumetee S, Reardon DA, Friedman AH, Bigner DD, Friedman HS. Chemotherapy and novel therapeutic approaches in malignant gliomas. Front Biosci. 2005;10:2645–2668. - PubMed
-
- Giese A, Bjerkvig R, Berens ME, Westphal M. Cost of migration: invasion of malignant gliomas and implications for treatment. J Clin Oncol. 2003;21:1624–1636. - PubMed
-
- Lefranc F, Brotchi J, Kiss R. Possible future issues in the treatment of GBMs: special emphasis on cell migration and the resistance of migrating glioblastoma cells to apoptosis. J Clin Oncol. 2005;23:2411–2422. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

