Estrogen plus progestin and breast cancer incidence and mortality in the Women's Health Initiative Observational Study
- PMID: 23543779
- PMCID: PMC3691942
- DOI: 10.1093/jnci/djt043
Estrogen plus progestin and breast cancer incidence and mortality in the Women's Health Initiative Observational Study
Abstract
Background: In the Women's Health Initiative (WHI) randomized trial, estrogen plus progestin increased both breast cancer incidence and mortality. In contrast, most observational studies associate estrogen plus progestin with favorable prognosis breast cancers. To address differences, a cohort of WHI observational study participants with characteristics similar to the WHI clinical trial was studied.
Methods: We identified 41 449 postmenopausal women with no prior hysterectomy and mammogram negative within 2 years who were either not hormone users (n = 25 328) or estrogen and progestin users (n = 16 121). Multivariable-adjusted Cox proportional hazard regression was used to calculate hazard ratios (HRs) with 95% confidence intervals (CI). All statistical tests were two-sided.
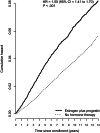
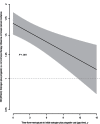
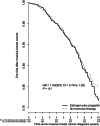
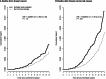
Results: After a mean of 11.3 (SD = 3.1) years, with 2236 breast cancers, incidence was higher in estrogen plus progestin users than in nonusers (0.60% vs 0.42%, annualized rate, respectively; HR = 1.55, 95% CI = 1.41 to 1.70, P < .001). Women initiating hormone therapy closer to menopause had higher breast cancer risk with linear diminishing influence as time from menopause increased (P < .001). Survival after breast cancer, measured from diagnosis, was similar in combined hormone therapy users and nonusers (HR = 1.03, 95% CI = 0.79 to 1.35). On a population basis, there were somewhat more deaths from breast cancer, measured from cohort entry (HR = 1.32, 95% CI = 0.90 to 1.93, P = .15), and more all-cause deaths after breast cancer (HR = 1.65, 95% CI = 1.29 to 2.12, P < .001) in estrogen plus progestin users than in nonusers.
Conclusions: Consistent with WHI randomized trial findings, estrogen plus progestin use is associated with increased breast cancer incidence. Because prognosis after diagnosis on combined hormone therapy is similar to that of nonusers, increased breast cancer mortality can be expected.
Figures
Comment in
-
The effect of estrogen plus progestin hormone therapy on breast cancer mortality: still unresolved.J Natl Cancer Inst. 2013 Apr 17;105(8):513-4. doi: 10.1093/jnci/djt058. Epub 2013 Mar 29. J Natl Cancer Inst. 2013. PMID: 23543780 No abstract available.
-
Re: Estrogen plus progestin and breast cancer incidence and mortality in the women's health initiative observational study.J Natl Cancer Inst. 2014 Feb;106(2):djt372. doi: 10.1093/jnci/djt372. Epub 2013 Dec 22. J Natl Cancer Inst. 2014. PMID: 24363446 No abstract available.
Similar articles
-
Changing concepts: Menopausal hormone therapy and breast cancer.J Natl Cancer Inst. 2012 Apr 4;104(7):517-27. doi: 10.1093/jnci/djs014. Epub 2012 Mar 16. J Natl Cancer Inst. 2012. PMID: 22427684 Free PMC article. Review.
-
Lung cancer among postmenopausal women treated with estrogen alone in the women's health initiative randomized trial.J Natl Cancer Inst. 2010 Sep 22;102(18):1413-21. doi: 10.1093/jnci/djq285. Epub 2010 Aug 13. J Natl Cancer Inst. 2010. PMID: 20709992 Free PMC article. Clinical Trial.
-
Breast Cancer and Menopausal Hormone Therapy by Race/Ethnicity and Body Mass Index.J Natl Cancer Inst. 2015 Nov 5;108(2):djv327. doi: 10.1093/jnci/djv327. Print 2016 Feb. J Natl Cancer Inst. 2015. PMID: 26546117 Free PMC article. Review.
-
Re: Estrogen plus progestin and breast cancer incidence and mortality in the women's health initiative observational study.J Natl Cancer Inst. 2014 Feb;106(2):djt372. doi: 10.1093/jnci/djt372. Epub 2013 Dec 22. J Natl Cancer Inst. 2014. PMID: 24363446 No abstract available.
-
The Women's Health Initiative randomized trials of menopausal hormone therapy and breast cancer: findings in context.Menopause. 2023 Apr 1;30(4):454-461. doi: 10.1097/GME.0000000000002154. Epub 2023 Jan 22. Menopause. 2023. PMID: 36727752
Cited by
-
Classical and Non-Classical Progesterone Signaling in Breast Cancers.Cancers (Basel). 2020 Aug 27;12(9):2440. doi: 10.3390/cancers12092440. Cancers (Basel). 2020. PMID: 32867363 Free PMC article. Review.
-
Gene expression trend changes in breast cancer populations over two decades: insights from The Cancer Genome Atlas database.Hereditas. 2022 Mar 22;159(1):18. doi: 10.1186/s41065-022-00230-3. Hereditas. 2022. PMID: 35317849 Free PMC article.
-
Breast Cancer Risk Factors and Survival by Tumor Subtype: Pooled Analyses from the Breast Cancer Association Consortium.Cancer Epidemiol Biomarkers Prev. 2021 Apr;30(4):623-642. doi: 10.1158/1055-9965.EPI-20-0924. Epub 2021 Jan 26. Cancer Epidemiol Biomarkers Prev. 2021. PMID: 33500318 Free PMC article.
-
Botanicals and Their Bioactive Phytochemicals for Women's Health.Pharmacol Rev. 2016 Oct;68(4):1026-1073. doi: 10.1124/pr.115.010843. Pharmacol Rev. 2016. PMID: 27677719 Free PMC article. Review.
-
Screening for Chemical Contributions to Breast Cancer Risk: A Case Study for Chemical Safety Evaluation.Environ Health Perspect. 2015 Dec;123(12):1255-64. doi: 10.1289/ehp.1408337. Epub 2015 Jun 2. Environ Health Perspect. 2015. PMID: 26032647 Free PMC article. Review.
References
-
- Chlebowski RT, Hendrix SL, Langer RD, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women’s Health Initiative randomized trial. JAMA. 2003; 289(24):3243–3253 - PubMed
-
- Colditz GA, Hankinson SE, Hunter DJ, et al. The use of estrogens and progestins and the risk of breast cancer in postmenopausal women. N Engl J Med. 1995; 332(24):1589–1593 - PubMed
-
- Bergkvist L, Adami HO, Persson J, et al. Prognosis after breast cancer diagnosis in women exposed to estrogen and estrogen-progestogen replacement therapy. Am J Epidemiol. 1989; 130(2):221–228 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

