An antisense oligonucleotide against SOD1 delivered intrathecally for patients with SOD1 familial amyotrophic lateral sclerosis: a phase 1, randomised, first-in-man study
- PMID: 23541756
- PMCID: PMC3712285
- DOI: 10.1016/S1474-4422(13)70061-9
An antisense oligonucleotide against SOD1 delivered intrathecally for patients with SOD1 familial amyotrophic lateral sclerosis: a phase 1, randomised, first-in-man study
Erratum in
- Lancet Neurol. 2013 May;12(5):423
Abstract
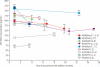
Background: Mutations in SOD1 cause 13% of familial amyotrophic lateral sclerosis. In the SOD1 Gly93Ala rat model of amyotrophic lateral sclerosis, the antisense oligonucleotide ISIS 333611 delivered to CSF decreased SOD1 mRNA and protein concentrations in spinal cord tissue and prolonged survival. We aimed to assess the safety, tolerability, and pharmacokinetics of ISIS 333611 after intrathecal administration in patients with SOD1-related familial amyotrophic lateral sclerosis.
Methods: In this randomised, placebo-controlled, phase 1 trial, we delivered ISIS 333611 by intrathecal infusion using an external pump over 11·5 h at increasing doses (0·15 mg, 0·50 mg, 1·50 mg, 3·00 mg) to four cohorts of eight patients with SOD1-positive amyotrophic lateral sclerosis (six patients assigned to ISIS 333611, two to placebo in each cohort). We did the randomisation with a web-based system, assigning patients in blocks of four. Patients and investigators were masked to treatment assignment. Participants were allowed to re-enrol in subsequent cohorts. Our primary objective was to assess the safety and tolerability of ISIS 333611. Assessments were done during infusion and over 28 days after infusion. This study was registered with Clinicaltrials.gov, number NCT01041222.
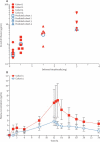
Findings: Seven of eight (88%) patients in the placebo group versus 20 of 24 (83%) in the ISIS 333611 group had adverse events. The most common events were post-lumbar puncture syndrome (3/8 [38%] vs 8/24 [33%]), back pain (4/8 [50%] vs 4/24 [17%]), and nausea (0/8 [0%] vs 3/24 [13%]). We recorded no dose-limiting toxic effects or any safety or tolerability concerns related to ISIS 333611. No serious adverse events occurred in patients given ISIS 333611. Re-enrolment and re-treatment were also well tolerated.
Interpretation: This trial is the first clinical study of intrathecal delivery of an antisense oligonucleotide. ISIS 333611 was well tolerated when administered as an intrathecal infusion. Antisense oligonucleotides delivered to the CNS might be a feasible treatment for neurological disorders.
Funding: The ALS Association, Muscular Dystrophy Association, Isis Pharmaceuticals.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures
Comment in
-
Antisense makes sense for amyotrophic lateral sclerosis.Lancet Neurol. 2013 May;12(5):416-7. doi: 10.1016/S1474-4422(13)70059-0. Epub 2013 Mar 29. Lancet Neurol. 2013. PMID: 23541757 No abstract available.
-
Why antisense could make sense for neurodegeneration.J Neurol. 2017 Jul;264(7):1542-1544. doi: 10.1007/s00415-017-8515-y. J Neurol. 2017. PMID: 28516330 Free PMC article. No abstract available.
Similar articles
-
Phase 1-2 Trial of Antisense Oligonucleotide Tofersen for SOD1 ALS.N Engl J Med. 2020 Jul 9;383(2):109-119. doi: 10.1056/NEJMoa2003715. N Engl J Med. 2020. PMID: 32640130 Clinical Trial.
-
Trial of Antisense Oligonucleotide Tofersen for SOD1 ALS.N Engl J Med. 2022 Sep 22;387(12):1099-1110. doi: 10.1056/NEJMoa2204705. N Engl J Med. 2022. PMID: 36129998 Clinical Trial.
-
Safety and efficacy of rasagiline as an add-on therapy to riluzole in patients with amyotrophic lateral sclerosis: a randomised, double-blind, parallel-group, placebo-controlled, phase 2 trial.Lancet Neurol. 2018 Aug;17(8):681-688. doi: 10.1016/S1474-4422(18)30176-5. Epub 2018 Jun 19. Lancet Neurol. 2018. PMID: 29934198 Clinical Trial.
-
Recent Progress of Antisense Oligonucleotide Therapy for Superoxide-Dismutase-1-Mutated Amyotrophic Lateral Sclerosis: Focus on Tofersen.Genes (Basel). 2024 Oct 20;15(10):1342. doi: 10.3390/genes15101342. Genes (Basel). 2024. PMID: 39457466 Free PMC article. Review.
-
[Amyotrophic lateral sclerosis with the SOD1 mutations].Rinsho Shinkeigaku. 2008 Nov;48(11):966-9. doi: 10.5692/clinicalneurol.48.966. Rinsho Shinkeigaku. 2008. PMID: 19198133 Review. Japanese.
Cited by
-
Discovery of Novel Inhibitors against ALS-Related SOD1(A4V) Aggregation through the Screening of a Chemical Library Using Differential Scanning Fluorimetry (DSF).Pharmaceuticals (Basel). 2024 Sep 27;17(10):1286. doi: 10.3390/ph17101286. Pharmaceuticals (Basel). 2024. PMID: 39458929 Free PMC article.
-
Antisense oligonucleotide therapy in a humanized mouse model of MECP2 duplication syndrome.Sci Transl Med. 2021 Mar 3;13(583):eaaz7785. doi: 10.1126/scitranslmed.aaz7785. Sci Transl Med. 2021. PMID: 33658357 Free PMC article.
-
Patients with Amyotrophic Lateral Sclerosis Have High Interest in and Limited Access to Genetic Testing.J Genet Couns. 2017 Jun;26(3):604-611. doi: 10.1007/s10897-016-0034-y. Epub 2016 Oct 20. J Genet Couns. 2017. PMID: 27761850
-
Beyond the Traditional Clinical Trials for Amyotrophic Lateral Sclerosis and The Future Impact of Gene Therapy.J Neuromuscul Dis. 2021;8(1):25-38. doi: 10.3233/JND-200531. J Neuromuscul Dis. 2021. PMID: 33074186 Free PMC article. Review.
-
Tofersen for SOD1 ALS.Neurodegener Dis Manag. 2024;14(5):149-160. doi: 10.1080/17582024.2024.2402216. Epub 2024 Sep 27. Neurodegener Dis Manag. 2024. PMID: 39330700 Free PMC article. Review.
References
-
- Bras J, Guerreiro R, Hardy J. Use of next-generation sequencing and other whole-genome strategies to dissect neurological disease. Nat Rev Neurosci. 2012;13(7):453–64. - PubMed
-
- Miller TM, Smith RA, Kordasiewicz H, Kaspar BK. Gene-targeted therapies for the central nervous system. Arch Neurol. 2008;65(4):447–51. - PubMed
-
- Bennett CF, Swayze EE. RNA targeting therapeutics: molecular mechanisms of antisense oligonucleotides as a therapeutic platform. Annu Rev Pharmacol Toxicol. 2010;50:259–93. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

