Restoring immune function of tumor-specific CD4+ T cells during recurrence of melanoma
- PMID: 23536636
- PMCID: PMC3633733
- DOI: 10.4049/jimmunol.1300271
Restoring immune function of tumor-specific CD4+ T cells during recurrence of melanoma
Abstract
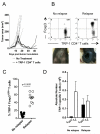
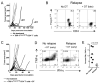
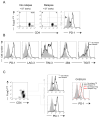
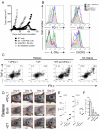
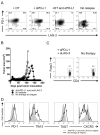
Recurrent solid malignancies are often refractory to standard therapies. Although adoptive T cell transfer may benefit select individuals, the majority of patients succumb to their disease. To address this important clinical dilemma, we developed a mouse melanoma model in which initial regression of advanced disease was followed by tumor recurrence. During recurrence, Foxp3(+) tumor-specific CD4(+) T cells became PD-1(+) and represented >60% of the tumor-specific CD4(+) T cells in the host. Concomitantly, tumor-specific CD4(+) T effector cells showed traits of chronic exhaustion, as evidenced by their high expression of the PD-1, TIM-3, 2B4, TIGIT, and LAG-3 inhibitory molecules. Although blockade of the PD-1/PD-L1 pathway with anti-PD-L1 Abs or depletion of tumor-specific regulatory T cells (Tregs) alone failed to reverse tumor recurrence, the combination of PD-L1 blockade with tumor-specific Treg depletion effectively mediated disease regression. Furthermore, blockade with a combination of anti-PD-L1 and anti-LAG-3 Abs overcame the requirement to deplete tumor-specific Tregs. In contrast, successful treatment of primary melanoma with adoptive cell therapy required only Treg depletion or Ab therapy, underscoring the differences in the characteristics of treatment between primary and relapsing cancer. These data highlight the need for preclinical development of combined immunotherapy approaches specifically targeting recurrent disease.
Figures
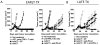
Similar articles
-
PD-L1-Independent Mechanisms Control the Resistance of Melanoma to CD4+ T Cell Adoptive Immunotherapy.J Immunol. 2018 May 1;200(9):3304-3311. doi: 10.4049/jimmunol.1701617. Epub 2018 Mar 30. J Immunol. 2018. PMID: 29602773
-
Blockade of LAG-3 in PD-L1-Deficient Mice Enhances Clearance of Blood Stage Malaria Independent of Humoral Responses.Front Immunol. 2021 Jan 14;11:576743. doi: 10.3389/fimmu.2020.576743. eCollection 2020. Front Immunol. 2021. PMID: 33519801 Free PMC article.
-
Immune suppression in premalignant respiratory papillomas: enriched functional CD4+Foxp3+ regulatory T cells and PD-1/PD-L1/L2 expression.Clin Cancer Res. 2012 Apr 1;18(7):1925-35. doi: 10.1158/1078-0432.CCR-11-2941. Epub 2012 Feb 9. Clin Cancer Res. 2012. PMID: 22322668 Free PMC article.
-
Immune checkpoint proteins: a new therapeutic paradigm for cancer--preclinical background: CTLA-4 and PD-1 blockade.Semin Oncol. 2010 Oct;37(5):430-9. doi: 10.1053/j.seminoncol.2010.09.005. Semin Oncol. 2010. PMID: 21074057 Review.
-
The PD-1 pathway in tolerance and autoimmunity.Immunol Rev. 2010 Jul;236:219-42. doi: 10.1111/j.1600-065X.2010.00923.x. Immunol Rev. 2010. PMID: 20636820 Free PMC article. Review.
Cited by
-
Challenges of Using High-Dose Fractionation Radiotherapy in Combination Therapy.Front Oncol. 2016 Jun 30;6:165. doi: 10.3389/fonc.2016.00165. eCollection 2016. Front Oncol. 2016. PMID: 27446811 Free PMC article. Review.
-
LAG-3: from molecular functions to clinical applications.J Immunother Cancer. 2020 Sep;8(2):e001014. doi: 10.1136/jitc-2020-001014. J Immunother Cancer. 2020. PMID: 32929051 Free PMC article. Review.
-
T-cell exhaustion in the tumor microenvironment.Cell Death Dis. 2015 Jun 18;6(6):e1792. doi: 10.1038/cddis.2015.162. Cell Death Dis. 2015. PMID: 26086965 Free PMC article. Review.
-
Oncogenic BRAFV600E Governs Regulatory T-cell Recruitment during Melanoma Tumorigenesis.Cancer Res. 2018 Sep 1;78(17):5038-5049. doi: 10.1158/0008-5472.CAN-18-0365. Epub 2018 Jul 19. Cancer Res. 2018. PMID: 30026331 Free PMC article.
-
Chronic thoracic spinal cord injury impairs CD8+ T-cell function by up-regulating programmed cell death-1 expression.J Neuroinflammation. 2014 Apr 1;11:65. doi: 10.1186/1742-2094-11-65. J Neuroinflammation. 2014. PMID: 24690491 Free PMC article.
References
-
- Quezada SA, Simpson TR, Peggs KS, Merghoub T, Vider J, Fan X, Blasberg R, Yagita H, Muranski P, Antony PA, Restifo NP, Allison JP. Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med. 2010;207:637–650. - PMC - PubMed
-
- Muranski P, Borman ZA, Kerkar SP, Klebanoff CA, Ji Y, Sanchez-Perez L, Sukumar M, Reger RN, Yu Z, Kern SJ, Roychoudhuri R, Ferreyra GA, Shen W, Durum SK, Feigenbaum L, Palmer DC, Antony PA, Chan CC, Laurence A, Danner RL, Gattinoni L, Restifo NP. Th17 cells are long lived and retain a stem cell-like molecular signature. Immunity. 2011;35:972–985. - PMC - PubMed
-
- Landsberg J, Kohlmeyer J, Renn M, Bald T, Rogava M, Cron M, Fatho M, Lennerz V, Wolfel T, Holzel M, Tuting T. Melanomas resist T-cell therapy through inflammation-induced reversible dedifferentiation. Nature. 2012 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

