An initial and rapid step of lytic granule secretion precedes microtubule organizing center polarization at the cytotoxic T lymphocyte/target cell synapse
- PMID: 23536289
- PMCID: PMC3625254
- DOI: 10.1073/pnas.1218640110
An initial and rapid step of lytic granule secretion precedes microtubule organizing center polarization at the cytotoxic T lymphocyte/target cell synapse
Abstract
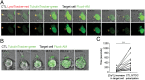
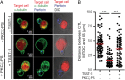
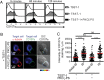
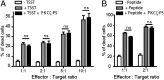
It is presently assumed that lethal hit delivery by cytotoxic T lymphocytes (CTLs) is mechanistically linked to centrosome polarization toward target cells, leading to dedicated release of lytic granules within a confined secretory domain. Here we provide three lines of evidence showing that this mechanism might not apply as a general paradigm for lethal hit delivery. First, in CTLs stimulated with immobilized peptide-MHC complexes, lytic granules and microtubule organizing center localization into synaptic areas are spatio-temporally dissociated, as detected by total internal reflection fluorescence microscopy. Second, in many CTL/target cell conjugates, lytic granule secretion precedes microtubule polarization and can be detected during the first minute after cell-cell contact. Third, inhibition of microtubule organizing center and centrosome polarization impairs neither lytic granule release at the CTL synapse nor killing efficiency. Our results broaden current views of CTL biology by revealing an extremely rapid step of lytic granule secretion and by showing that microtubule organizing center polarization is dispensable for efficient lethal hit delivery.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Centrosome polarization delivers secretory granules to the immunological synapse.Nature. 2006 Sep 28;443(7110):462-5. doi: 10.1038/nature05071. Nature. 2006. PMID: 17006514
-
The strength of T cell receptor signal controls the polarization of cytotoxic machinery to the immunological synapse.Immunity. 2009 Oct 16;31(4):621-31. doi: 10.1016/j.immuni.2009.08.024. Immunity. 2009. PMID: 19833087 Free PMC article.
-
Secretory mechanisms in cell-mediated cytotoxicity.Annu Rev Cell Dev Biol. 2007;23:495-517. doi: 10.1146/annurev.cellbio.23.090506.123521. Annu Rev Cell Dev Biol. 2007. PMID: 17506701 Review.
-
Kinetics of early T cell receptor signaling regulate the pathway of lytic granule delivery to the secretory domain.Immunity. 2009 Oct 16;31(4):632-42. doi: 10.1016/j.immuni.2009.09.004. Immunity. 2009. PMID: 19833088 Free PMC article.
-
Signals Controlling Lytic Granule Polarization at the Cytotoxic Immune Synapse.Front Immunol. 2018 Feb 20;9:307. doi: 10.3389/fimmu.2018.00307. eCollection 2018. Front Immunol. 2018. PMID: 29515593 Free PMC article. Review.
Cited by
-
Perforin and granzymes: function, dysfunction and human pathology.Nat Rev Immunol. 2015 Jun;15(6):388-400. doi: 10.1038/nri3839. Nat Rev Immunol. 2015. PMID: 25998963 Review.
-
Mechanically active integrins target lytic secretion at the immune synapse to facilitate cellular cytotoxicity.Nat Commun. 2022 Jun 9;13(1):3222. doi: 10.1038/s41467-022-30809-3. Nat Commun. 2022. PMID: 35680882 Free PMC article.
-
Centrioles control the capacity, but not the specificity, of cytotoxic T cell killing.Proc Natl Acad Sci U S A. 2020 Feb 25;117(8):4310-4319. doi: 10.1073/pnas.1913220117. Epub 2020 Feb 10. Proc Natl Acad Sci U S A. 2020. PMID: 32041868 Free PMC article.
-
Chimeric antigen receptor T cells form nonclassical and potent immune synapses driving rapid cytotoxicity.Proc Natl Acad Sci U S A. 2018 Feb 27;115(9):E2068-E2076. doi: 10.1073/pnas.1716266115. Epub 2018 Feb 12. Proc Natl Acad Sci U S A. 2018. PMID: 29440406 Free PMC article.
-
Molecular mechanisms and functional implications of polarized actin remodeling at the T cell immunological synapse.Cell Mol Life Sci. 2015 Feb;72(3):537-556. doi: 10.1007/s00018-014-1760-7. Epub 2014 Oct 30. Cell Mol Life Sci. 2015. PMID: 25355055 Free PMC article. Review.
References
-
- Law RH, et al. The structural basis for membrane binding and pore formation by lymphocyte perforin. Nature. 2010;468(7322):447–451. - PubMed
-
- de Saint Basile G, Ménasché G, Fischer A. Molecular mechanisms of biogenesis and exocytosis of cytotoxic granules. Nat Rev Immunol. 2010;10(8):568–579. - PubMed
-
- Purbhoo MA, Irvine DJ, Huppa JB, Davis MM. T cell killing does not require the formation of a stable mature immunological synapse. Nat Immunol. 2004;5(5):524–530. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

