Targeted ablation of miR-21 decreases murine eosinophil progenitor cell growth
- PMID: 23533623
- PMCID: PMC3606295
- DOI: 10.1371/journal.pone.0059397
Targeted ablation of miR-21 decreases murine eosinophil progenitor cell growth
Abstract
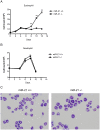
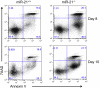
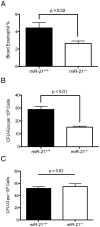
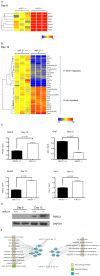
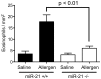
MiR-21 is one of the most up-regulated miRNAs in multiple allergic diseases associated with eosinophilia and has been shown to positively correlate with eosinophil levels. Herein, we show that miR-21 is up-regulated during IL-5-driven eosinophil differentiation from progenitor cells in vitro. Targeted ablation of miR-21 leads to reduced eosinophil progenitor cell growth. Furthermore, miR-21(-/-) eosinophil progenitor cells have increased apoptosis as indicated by increased levels of annexin V positivity compared to miR-21(+/+) eosinophil progenitor cells. Indeed, miR-21(-/-) mice have reduced blood eosinophil levels in vivo and reduced eosinophil colony forming unit capacity in the bone marrow. Using gene expression microarray analysis, we identified dysregulation of genes involved in cell proliferation (e,g, Ms4a3, Grb7), cell cycle and immune response as the most significant pathways affected by miR-21 in eosinophil progenitors. These results demonstrate that miR-21 can regulate the development of eosinophils by influencing eosinophil progenitor cell growth. Our findings have identified one of the first miRNAs with a role in regulating eosinophil development.
Conflict of interest statement
Figures

Similar articles
-
MiR-223 deficiency increases eosinophil progenitor proliferation.J Immunol. 2013 Feb 15;190(4):1576-82. doi: 10.4049/jimmunol.1202897. Epub 2013 Jan 16. J Immunol. 2013. PMID: 23325891 Free PMC article.
-
Paired immunoglobulin-like receptor A is an intrinsic, self-limiting suppressor of IL-5-induced eosinophil development.Nat Immunol. 2014 Jan;15(1):36-44. doi: 10.1038/ni.2757. Epub 2013 Nov 10. Nat Immunol. 2014. PMID: 24212998 Free PMC article.
-
Generation of eosinophils from cryopreserved murine bone marrow cells.PLoS One. 2014 Dec 31;9(12):e116141. doi: 10.1371/journal.pone.0116141. eCollection 2014. PLoS One. 2014. PMID: 25551463 Free PMC article.
-
Expression profiling of differentiating eosinophils in bone marrow cultures predicts functional links between microRNAs and their target mRNAs.PLoS One. 2014 May 13;9(5):e97537. doi: 10.1371/journal.pone.0097537. eCollection 2014. PLoS One. 2014. PMID: 24824797 Free PMC article.
-
The CCR3 receptor is involved in eosinophil differentiation and is up-regulated by Th2 cytokines in CD34+ progenitor cells.J Immunol. 2003 Jan 1;170(1):537-47. doi: 10.4049/jimmunol.170.1.537. J Immunol. 2003. PMID: 12496441
Cited by
-
MicroRNAs: Potential Biomarkers and Targets of Therapy in Allergic Diseases?Arch Immunol Ther Exp (Warsz). 2019 Aug;67(4):213-223. doi: 10.1007/s00005-019-00547-4. Epub 2019 May 28. Arch Immunol Ther Exp (Warsz). 2019. PMID: 31139837 Free PMC article. Review.
-
MicroRNA-21 maintains hematopoietic stem cell homeostasis through sustaining the NF-κB signaling pathway in mice.Haematologica. 2021 Feb 1;106(2):412-423. doi: 10.3324/haematol.2019.236927. Haematologica. 2021. PMID: 31974197 Free PMC article.
-
miR-21 Might be Involved in Breast Cancer Promotion and Invasion Rather than in Initial Events of Breast Cancer Development.Mol Diagn Ther. 2016 Apr;20(2):97-110. doi: 10.1007/s40291-016-0186-3. Mol Diagn Ther. 2016. PMID: 26891730 Review.
-
Construction and analysis of regulatory genetic networks in cervical cancer based on involved microRNAs, target genes, transcription factors and host genes.Oncol Lett. 2014 Apr;7(4):1279-1283. doi: 10.3892/ol.2014.1814. Epub 2014 Jan 20. Oncol Lett. 2014. PMID: 24944708 Free PMC article.
-
Myeloid-derived microRNAs, miR-223, miR27a, and miR-652, are dominant players in myeloid regulation.Biomed Res Int. 2014;2014:870267. doi: 10.1155/2014/870267. Epub 2014 Aug 11. Biomed Res Int. 2014. PMID: 25177699 Free PMC article. Review.
References
-
- Hogan SP, Rosenberg HF, Moqbel R, Phipps S, Foster PS, et al. (2008) Eosinophils: biological properties and role in health and disease. Clin Exp Allergy 38: 709–750. - PubMed
-
- Kouro T, Takatsu K (2009) IL-5- and eosinophil-mediated inflammation: from discovery to therapy. Int Immunol 21: 1303–1309. - PubMed
-
- Rosas M, Dijkers PF, Lindemans CL, Lammers JW, Koenderman L, et al. (2006) IL-5-mediated eosinophil survival requires inhibition of GSK-3 and correlates with beta-catenin relocalization. J Leukoc Biol 80: 186–195. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

