Reporting of analyses from randomized controlled trials with multiple arms: a systematic review
- PMID: 23531230
- PMCID: PMC3621416
- DOI: 10.1186/1741-7015-11-84
Reporting of analyses from randomized controlled trials with multiple arms: a systematic review
Abstract
Background: Multiple-arm randomized trials can be more complex in their design, data analysis, and result reporting than two-arm trials. We conducted a systematic review to assess the reporting of analyses in reports of randomized controlled trials (RCTs) with multiple arms.
Methods: The literature in the MEDLINE database was searched for reports of RCTs with multiple arms published in 2009 in the core clinical journals. Two reviewers extracted data using a standardized extraction form.
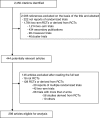
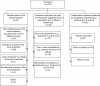
Results: In total, 298 reports were identified. Descriptions of the baseline characteristics and outcomes per group were missing in 45 reports (15.1%) and 48 reports (16.1%), respectively. More than half of the articles (n = 171, 57.4%) reported that a planned global test comparison was used (that is, assessment of the global differences between all groups), but 67 (39.2%) of these 171 articles did not report details of the planned analysis. Of the 116 articles reporting a global comparison test, 12 (10.3%) did not report the analysis as planned. In all, 60% of publications (n = 180) described planned pairwise test comparisons (that is, assessment of the difference between two groups), but 20 of these 180 articles (11.1%) did not report the pairwise test comparisons. Of the 204 articles reporting pairwise test comparisons, the comparisons were not planned for 44 (21.6%) of them. Less than half the reports (n = 137; 46%) provided baseline and outcome data per arm and reported the analysis as planned.
Conclusions: Our findings highlight discrepancies between the planning and reporting of analyses in reports of multiple-arm trials.
Figures
Similar articles
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
A systematic review of comparisons between protocols or registrations and full reports in primary biomedical research.BMC Med Res Methodol. 2018 Jan 11;18(1):9. doi: 10.1186/s12874-017-0465-7. BMC Med Res Methodol. 2018. PMID: 29325533 Free PMC article.
-
Behavioral and Pharmacotherapy Weight Loss Interventions to Prevent Obesity-Related Morbidity and Mortality in Adults: An Updated Systematic Review for the U.S. Preventive Services Task Force [Internet].Rockville (MD): Agency for Healthcare Research and Quality (US); 2018 Sep. Report No.: 18-05239-EF-1. Rockville (MD): Agency for Healthcare Research and Quality (US); 2018 Sep. Report No.: 18-05239-EF-1. PMID: 30354042 Free Books & Documents. Review.
-
Current practice in analysing and reporting binary outcome data-a review of randomised controlled trial reports.BMC Med. 2020 Jun 8;18(1):147. doi: 10.1186/s12916-020-01598-7. BMC Med. 2020. PMID: 32507111 Free PMC article. Review.
-
Reporting of safety results in published reports of randomized controlled trials.Arch Intern Med. 2009 Oct 26;169(19):1756-61. doi: 10.1001/archinternmed.2009.306. Arch Intern Med. 2009. PMID: 19858432
Cited by
-
Evaluation of a multi-arm multi-stage Bayesian design for phase II drug selection trials - an example in hemato-oncology.BMC Med Res Methodol. 2016 Jun 2;16:67. doi: 10.1186/s12874-016-0166-7. BMC Med Res Methodol. 2016. PMID: 27250349 Free PMC article. Clinical Trial.
-
Mindfulness-Based Intervention for Caregivers of Frail Older Chinese Adults: A Study Protocol.Int J Environ Res Public Health. 2022 Apr 29;19(9):5447. doi: 10.3390/ijerph19095447. Int J Environ Res Public Health. 2022. PMID: 35564839 Free PMC article.
-
Optimal individualized decision rules from a multi-arm trial: A comparison of methods and an application to tailoring inter-donation intervals among blood donors in the UK.Stat Methods Med Res. 2020 Nov;29(11):3113-3134. doi: 10.1177/0962280220920669. Epub 2020 May 8. Stat Methods Med Res. 2020. PMID: 32380893 Free PMC article.
-
Housing First Improves Adherence to Antipsychotic Medication Among Formerly Homeless Adults With Schizophrenia: Results of a Randomized Controlled Trial.Schizophr Bull. 2017 Jul 1;43(4):852-861. doi: 10.1093/schbul/sbw136. Schizophr Bull. 2017. PMID: 27665002 Free PMC article. Clinical Trial.
-
The Baby's First Bites RCT: Evaluating a Vegetable-Exposure and a Sensitive-Feeding Intervention in Terms of Child Health Outcomes and Maternal Feeding Behavior During Toddlerhood.J Nutr. 2022 Feb 8;152(2):386-398. doi: 10.1093/jn/nxab387. J Nutr. 2022. PMID: 34791320 Free PMC article. Clinical Trial.
References
-
- Cook RJ, Farewell VT. Multiplicity considerations in the design and analysis of clinical trials. J R Statistic Soc A. 1996;159(1):93–110. doi: 10.2307/2983471. - DOI
-
- Committee for Proprietary Medicinal Products. Points to consider on multiplicity issued in clinical trials. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guidelin....
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources