Generation of multi-leukemia antigen-specific T cells to enhance the graft-versus-leukemia effect after allogeneic stem cell transplant
- PMID: 23528871
- PMCID: PMC3867129
- DOI: 10.1038/leu.2013.66
Generation of multi-leukemia antigen-specific T cells to enhance the graft-versus-leukemia effect after allogeneic stem cell transplant
Abstract
Adoptive immunotherapy with ex vivo expanded T cells is a promising approach to prevent or treat leukemia. Myeloid leukemias express tumor-associated antigens (TAA) that induce antigen-specific cytotoxic T lymphocyte (CTL) responses in healthy individuals. We explored the feasibility of generating TAA-specific CTLs from stem cell donors of patients with myeloid leukemia to enhance the graft-versus-leukemia effect after stem cell transplantation. CTL lines were manufactured from peripheral blood of 10 healthy donors by stimulation with 15mer peptide libraries of five TAA (proteinase 3 (Pr3), preferentially expressed antigen in melanoma, Wilms tumor gene 1 (WT1), human neutrophil elastase (NE) and melanoma-associated antigen A3) known to be expressed in myeloid leukemias. All CTL lines responded to the mix of five TAA and were multi-specific as assessed by interferon-γ enzyme-linked immunospot. Although donors showed individual patterns of antigen recognition, all responded comparably to the TAAmix. Immunogenic peptides of WT1, Pr3 or NE could be identified by epitope mapping in all donor CTL lines. In vitro experiments showed recognition of partially human leukocyte antigen (HLA)-matched myeloid leukemia blasts. These findings support the development of a single clinical grade multi-tumor antigen-specific T-cell product from the stem cell source, capable of broad reactivity against myeloid malignancies for use in donor-recipient pairs without limitation to a certain HLA-type.
Conflict of interest statement
Figures
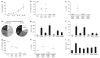
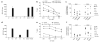

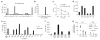
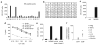
Similar articles
-
The in vitro generation of multi-tumor antigen-specific cytotoxic T cell clones: Candidates for leukemia adoptive immunotherapy following allogeneic stem cell transplantation.Mol Immunol. 2016 Sep;77:79-88. doi: 10.1016/j.molimm.2016.07.012. Epub 2016 Aug 1. Mol Immunol. 2016. PMID: 27490939
-
Generation of donor-derived Wilms tumor antigen 1-specific cytotoxic T lymphocytes with potent anti-leukemia activity for somatic cell therapy in children given haploidentical stem cell transplantation: a feasibility pre-clinical study.Cytotherapy. 2019 Sep;21(9):958-972. doi: 10.1016/j.jcyt.2019.06.007. Epub 2019 Jul 4. Cytotherapy. 2019. PMID: 31279696
-
PR1-specific cytotoxic T lymphocytes are relatively frequent in umbilical cord blood and can be effectively expanded to target myeloid leukemia.Cytotherapy. 2016 Aug;18(8):995-1001. doi: 10.1016/j.jcyt.2016.05.007. Cytotherapy. 2016. PMID: 27378343 Free PMC article.
-
T cell-mediated graft-versus-leukemia reactions after allogeneic stem cell transplantation.Cancer Immunol Immunother. 2005 Nov;54(11):1043-58. doi: 10.1007/s00262-005-0681-6. Epub 2005 May 11. Cancer Immunol Immunother. 2005. PMID: 15887014 Free PMC article. Review.
-
Minor histocompatibility antigens as targets of graft-versus-leukemia reactions.Curr Opin Hematol. 2002 Nov;9(6):497-502. doi: 10.1097/00062752-200211000-00005. Curr Opin Hematol. 2002. PMID: 12394171 Review.
Cited by
-
Adoptive T-cell therapy for hematological malignancies using T cells gene-modified to express tumor antigen-specific receptors.Int J Hematol. 2014 Feb;99(2):123-31. doi: 10.1007/s12185-013-1493-7. Epub 2013 Dec 19. Int J Hematol. 2014. PMID: 24352938 Review.
-
Preclinical Validation of an Advanced Therapy Medicinal Product Based on Cytotoxic T Lymphocytes Specific for Mutated Nucleophosmin (NPM1mut) for the Treatment of NPM1mut-Acute Myeloid Leukemia.Cancers (Basel). 2023 May 12;15(10):2731. doi: 10.3390/cancers15102731. Cancers (Basel). 2023. PMID: 37345068 Free PMC article.
-
Simultaneous in vitro generation of CD8 and CD4 T cells specific to three universal tumor associated antigens of WT1, survivin and TERT and adoptive T cell transfer for the treatment of acute myeloid leukemia.Oncotarget. 2017 Jul 4;8(27):44059-44072. doi: 10.18632/oncotarget.17212. Oncotarget. 2017. PMID: 28477011 Free PMC article.
-
Adoptive Immunotherapy For Leukemia With Ex vivo Expanded T Cells.Curr Drug Targets. 2017;18(3):271-280. doi: 10.2174/1389450117666160209143529. Curr Drug Targets. 2017. PMID: 26648070 Free PMC article. Review.
-
The generation and application of antigen-specific T cell therapies for cancer and viral-associated disease.Mol Ther. 2022 Jun 1;30(6):2130-2152. doi: 10.1016/j.ymthe.2022.02.002. Epub 2022 Feb 9. Mol Ther. 2022. PMID: 35149193 Free PMC article. Review.
References
-
- Barrett AJ. Understanding and harnessing the graft-versus-leukaemia effect. Br J Haematol. 2008;142:877–888. - PubMed
-
- Montagna D, Maccario R, Locatelli F, Montini E, Pagani S, Bonetti F, et al. Emergence of antitumor cytolytic T cells is associated with maintenance of hemato-logic remission in children with acute myeloid leukemia. Blood. 2006;108:3843–3850. - PubMed
-
- Burnett AK, Knapper S. Acute Myeloid Leukemia. In: Treleaven J, Barrett AJ, editors. Haematopoietic Stem Cell Transplantation in Clinical Practice. Elsevier; 2009.
-
- Porter DL, June CH. T-cell reconstitution and expansion after hematopoietic stem cell transplantation: T' it up! Bone Marrow Transplant. 2005;35:935–942. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

