Amotl2 interacts with LL5β, localizes to podosomes and regulates postsynaptic differentiation in muscle
- PMID: 23525008
- PMCID: PMC3672938
- DOI: 10.1242/jcs.121327
Amotl2 interacts with LL5β, localizes to podosomes and regulates postsynaptic differentiation in muscle
Abstract
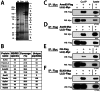
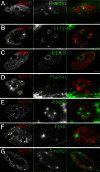
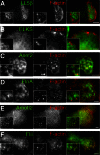
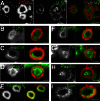
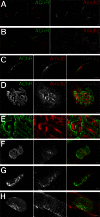
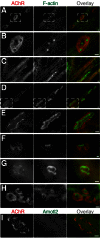
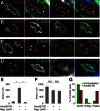
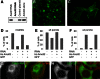
Neuromuscular junctions (NMJs) in mammalian skeletal muscle undergo a postnatal topological transformation from a simple oval plaque to a complex branched structure. We previously showed that podosomes, actin-rich adhesive organelles, promote the remodeling process, and demonstrated a key role for one podosome component, LL5β. To further investigate molecular mechanisms of postsynaptic maturation, we purified LL5β-associated proteins from myotubes and showed that three regulators of the actin cytoskeleton--Amotl2, Asef2 and Flii--interact with LL5β. These and other LL5β-interacting proteins are associated with conventional podosomes in macrophages and podosome-like invadopodia in fibroblasts, strengthening the close relationship between synaptic and non-synaptic podosomes. We then focused on Amotl2, showing that it is associated with synaptic podosomes in cultured myotubes and with NMJs in vivo. Depletion of Amotl2 in myotubes leads to increased size of synaptic podosomes and corresponding alterations in postsynaptic topology. Depletion of Amotl2 from fibroblasts disrupts invadopodia in these cells. These results demonstrate a role for Amotl2 in synaptic maturation and support the involvement of podosomes in this process.
Keywords: Acetylcholine receptor; Neuromuscular junction; Podosome.
Figures
Similar articles
-
Podosomes in muscle cells and their role in the remodeling of neuromuscular postsynaptic machinery.Eur J Cell Biol. 2014 Oct;93(10-12):478-85. doi: 10.1016/j.ejcb.2014.06.002. Epub 2014 Jun 16. Eur J Cell Biol. 2014. PMID: 25012928 Review.
-
Drebrin Regulates Acetylcholine Receptor Clustering and Organization of Microtubules at the Postsynaptic Machinery.Int J Mol Sci. 2021 Aug 30;22(17):9387. doi: 10.3390/ijms22179387. Int J Mol Sci. 2021. PMID: 34502296 Free PMC article.
-
LL5beta: a regulator of postsynaptic differentiation identified in a screen for synaptically enriched transcripts at the neuromuscular junction.J Cell Biol. 2005 Apr 25;169(2):355-66. doi: 10.1083/jcb.200411012. J Cell Biol. 2005. PMID: 15851520 Free PMC article.
-
Podosomes are present in a postsynaptic apparatus and participate in its maturation.Proc Natl Acad Sci U S A. 2009 Oct 27;106(43):18373-8. doi: 10.1073/pnas.0910391106. Epub 2009 Oct 12. Proc Natl Acad Sci U S A. 2009. PMID: 19822767 Free PMC article.
-
Maturation of a postsynaptic domain: Role of small Rho GTPases in organising nicotinic acetylcholine receptor aggregates at the vertebrate neuromuscular junction.J Anat. 2022 Nov;241(5):1148-1156. doi: 10.1111/joa.13526. Epub 2021 Aug 3. J Anat. 2022. PMID: 34342888 Free PMC article. Review.
Cited by
-
Angiomotin Family Members: Oncogenes or Tumor Suppressors?Int J Biol Sci. 2017 Jun 1;13(6):772-781. doi: 10.7150/ijbs.19603. eCollection 2017. Int J Biol Sci. 2017. PMID: 28656002 Free PMC article. Review.
-
Involvement of unconventional myosin VI in myoblast function and myotube formation.Histochem Cell Biol. 2015 Jul;144(1):21-38. doi: 10.1007/s00418-015-1322-6. Epub 2015 Apr 21. Histochem Cell Biol. 2015. PMID: 25896210 Free PMC article.
-
Luteolin activates M2 macrophages and suppresses M1 macrophages by upregulation of hsa_circ_0001326 in THP-1 derived macrophages.Bioengineered. 2022 Mar;13(3):5079-5090. doi: 10.1080/21655979.2022.2036897. Bioengineered. 2022. PMID: 35152837 Free PMC article.
-
Deciphering the involvement of the Hippo pathway co-regulators, YAP/TAZ in invadopodia formation and matrix degradation.Cell Death Dis. 2023 Apr 25;14(4):290. doi: 10.1038/s41419-023-05769-1. Cell Death Dis. 2023. PMID: 37185904 Free PMC article.
-
CLASP2-dependent microtubule capture at the neuromuscular junction membrane requires LL5β and actin for focal delivery of acetylcholine receptor vesicles.Mol Biol Cell. 2015 Mar 1;26(5):938-51. doi: 10.1091/mbc.E14-06-1158. Epub 2015 Jan 14. Mol Biol Cell. 2015. PMID: 25589673 Free PMC article.
References
-
- Adams D. H., Ruzehaji N., Strudwick X. L., Greenwood J. E., Campbell H. D., Arkell R., Cowin A. J. (2009). Attenuation of Flightless I, an actin-remodelling protein, improves burn injury repair via modulation of transforming growth factor (TGF)-beta1 and TGF-beta3. Br. J. Dermatol. 161, 326–336 10.1111/j.1365-2133.2009.09296.x - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

