Histone deacetylase 2 (HDAC2) regulates chromosome segregation and kinetochore function via H4K16 deacetylation during oocyte maturation in mouse
- PMID: 23516383
- PMCID: PMC3597510
- DOI: 10.1371/journal.pgen.1003377
Histone deacetylase 2 (HDAC2) regulates chromosome segregation and kinetochore function via H4K16 deacetylation during oocyte maturation in mouse
Abstract
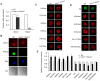
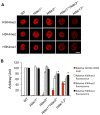
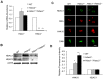
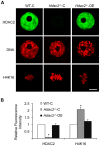
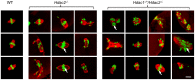
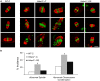
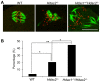
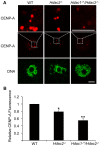
Changes in histone acetylation occur during oocyte development and maturation, but the role of specific histone deacetylases in these processes is poorly defined. We report here that mice harboring Hdac1(-/+)/Hdac2(-/-) or Hdac2(-/-) oocytes are infertile or sub-fertile, respectively. Depleting maternal HDAC2 results in hyperacetylation of H4K16 as determined by immunocytochemistry--normal deacetylation of other lysine residues of histone H3 or H4 is observed--and defective chromosome condensation and segregation during oocyte maturation occurs in a sub-population of oocytes. The resulting increased incidence of aneuploidy likely accounts for the observed sub-fertility of mice harboring Hdac2(-/-) oocytes. The infertility of mice harboring Hdac1(-/+)/Hdac2(-/-)oocytes is attributed to failure of those few eggs that properly mature to metaphase II to initiate DNA replication following fertilization. The increased amount of acetylated H4K16 likely impairs kinetochore function in oocytes lacking HDAC2 because kinetochores in mutant oocytes are less able to form cold-stable microtubule attachments and less CENP-A is located at the centromere. These results implicate HDAC2 as the major HDAC that regulates global histone acetylation during oocyte development and, furthermore, suggest HDAC2 is largely responsible for the deacetylation of H4K16 during maturation. In addition, the results provide additional support that histone deacetylation that occurs during oocyte maturation is critical for proper chromosome segregation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
HDAC11 promotes meiotic apparatus assembly during mouse oocyte maturation via decreasing H4K16 and α-tubulin acetylation.Cell Cycle. 2020 Feb;19(3):354-362. doi: 10.1080/15384101.2019.1711315. Epub 2020 Jan 7. Cell Cycle. 2020. PMID: 31910069 Free PMC article.
-
Histone methyltransferase SETD2 is required for meiotic maturation in mouse oocyte.J Cell Physiol. 2018 Jan;234(1):661-668. doi: 10.1002/jcp.26836. Epub 2018 Aug 5. J Cell Physiol. 2018. PMID: 30078214
-
In vitro maturation affects chromosome segregation, spindle morphology and acetylation of lysine 16 on histone H4 in horse oocytes.Reprod Fertil Dev. 2017 Apr;29(4):721-730. doi: 10.1071/RD15350. Reprod Fertil Dev. 2017. PMID: 26651296
-
HDAC1 and HDAC2 in mouse oocytes and preimplantation embryos: Specificity versus compensation.Cell Death Differ. 2016 Jul;23(7):1119-27. doi: 10.1038/cdd.2016.31. Epub 2016 Apr 15. Cell Death Differ. 2016. PMID: 27082454 Free PMC article. Review.
-
[The role of histone deacetylases 1/2 in regulating murine oogenesis].Sheng Li Xue Bao. 2021 Jun 25;73(3):527-534. Sheng Li Xue Bao. 2021. PMID: 34230954 Review. Chinese.
Cited by
-
Transcription and beyond: the role of mammalian class I lysine deacetylases.Chromosoma. 2014 Mar;123(1-2):67-78. doi: 10.1007/s00412-013-0441-x. Epub 2013 Oct 30. Chromosoma. 2014. PMID: 24170248 Free PMC article. Review.
-
Listening to mother: Long-term maternal effects in mammalian development.Mol Reprod Dev. 2020 Apr;87(4):399-408. doi: 10.1002/mrd.23336. Epub 2020 Mar 22. Mol Reprod Dev. 2020. PMID: 32202026 Free PMC article. Review.
-
Knockdown of RBBP7 unveils a requirement of histone deacetylation for CPC function in mouse oocytes.Cell Cycle. 2014;13(4):600-11. doi: 10.4161/cc.27410. Epub 2013 Dec 6. Cell Cycle. 2014. PMID: 24317350 Free PMC article.
-
Maternal histone acetyltransferase KAT8 is required for porcine preimplantation embryo development.Oncotarget. 2017 Oct 6;8(52):90250-90261. doi: 10.18632/oncotarget.21657. eCollection 2017 Oct 27. Oncotarget. 2017. PMID: 29163825 Free PMC article.
-
Melatonin ameliorates the advanced maternal age-associated meiotic defects in oocytes through the SIRT2-dependent H4K16 deacetylation pathway.Aging (Albany NY). 2020 Jan 24;12(2):1610-1623. doi: 10.18632/aging.102703. Epub 2020 Jan 24. Aging (Albany NY). 2020. PMID: 31980591 Free PMC article.
References
-
- Kouzarides T (2007) Chromatin modifications and their function. Cell 128: 693–705. - PubMed
-
- Iizuka M, Smith MM (2003) Functional consequences of histone modifications. Curr Opin Genet Dev 13: 154–160. - PubMed
-
- Jenuwein T, Allis CD (2001) Translating the histone code. Science 293: 1074–1080. - PubMed
-
- Brunmeir R, Lagger S, Seiser C (2009) Histone deacetylase HDAC1/HDAC2-controlled embryonic development and cell differentiation. Int J Dev Biol 53: 275–289. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

