Conformational biosensors reveal GPCR signalling from endosomes
- PMID: 23515162
- PMCID: PMC3835555
- DOI: 10.1038/nature12000
Conformational biosensors reveal GPCR signalling from endosomes
Abstract
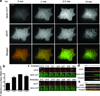
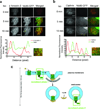
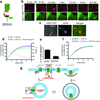
A long-held tenet of molecular pharmacology is that canonical signal transduction mediated by G-protein-coupled receptor (GPCR) coupling to heterotrimeric G proteins is confined to the plasma membrane. Evidence supporting this traditional view is based on analytical methods that provide limited or no subcellular resolution. It has been subsequently proposed that signalling by internalized GPCRs is restricted to G-protein-independent mechanisms such as scaffolding by arrestins, or GPCR activation elicits a discrete form of persistent G protein signalling, or that internalized GPCRs can indeed contribute to the acute G-protein-mediated response. Evidence supporting these various latter hypotheses is indirect or subject to alternative interpretation, and it remains unknown if endosome-localized GPCRs are even present in an active form. Here we describe the application of conformation-specific single-domain antibodies (nanobodies) to directly probe activation of the β2-adrenoceptor, a prototypical GPCR, and its cognate G protein, Gs (ref. 12), in living mammalian cells. We show that the adrenergic agonist isoprenaline promotes receptor and G protein activation in the plasma membrane as expected, but also in the early endosome membrane, and that internalized receptors contribute to the overall cellular cyclic AMP response within several minutes after agonist application. These findings provide direct support for the hypothesis that canonical GPCR signalling occurs from endosomes as well as the plasma membrane, and suggest a versatile strategy for probing dynamic conformational change in vivo.
Figures

Comment in
-
Cell biology: Receptor signals come in waves.Nature. 2013 Mar 28;495(7442):457-8. doi: 10.1038/nature12086. Epub 2013 Mar 20. Nature. 2013. PMID: 23515157 No abstract available.
-
Dispatches from the interior.Nat Methods. 2013 May;10(5):385. doi: 10.1038/nmeth.2468. Nat Methods. 2013. PMID: 23762907 No abstract available.
Similar articles
-
Allosteric coupling from G protein to the agonist-binding pocket in GPCRs.Nature. 2016 Jul 7;535(7610):182-6. doi: 10.1038/nature18324. Epub 2016 Jun 29. Nature. 2016. PMID: 27362234 Free PMC article.
-
Divergent agonist selectivity in activating β1- and β2-adrenoceptors for G-protein and arrestin coupling.Biochem J. 2011 Aug 15;438(1):191-202. doi: 10.1042/BJ20110374. Biochem J. 2011. PMID: 21561432
-
Internalized Receptor for Glucose-dependent Insulinotropic Peptide stimulates adenylyl cyclase on early endosomes.Biochem Pharmacol. 2016 Nov 15;120:33-45. doi: 10.1016/j.bcp.2016.09.009. Epub 2016 Sep 15. Biochem Pharmacol. 2016. PMID: 27641811
-
G protein-coupled receptor (GPCR) signaling via heterotrimeric G proteins from endosomes.J Biol Chem. 2015 Mar 13;290(11):6689-96. doi: 10.1074/jbc.R114.617951. Epub 2015 Jan 20. J Biol Chem. 2015. PMID: 25605726 Free PMC article. Review.
-
Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling.Pharmacol Rev. 2001 Mar;53(1):1-24. Pharmacol Rev. 2001. PMID: 11171937 Review.
Cited by
-
Functional proteomics, human genetics and cancer biology of GIPC family members.Exp Mol Med. 2013 Jun 7;45(6):e26. doi: 10.1038/emm.2013.49. Exp Mol Med. 2013. PMID: 23743496 Free PMC article. Review.
-
The synthetic progestin norgestrel modulates Nrf2 signaling and acts as an antioxidant in a model of retinal degeneration.Redox Biol. 2016 Dec;10:128-139. doi: 10.1016/j.redox.2016.10.002. Epub 2016 Oct 4. Redox Biol. 2016. PMID: 27744118 Free PMC article.
-
Minireview: Role of intracellular scaffolding proteins in the regulation of endocrine G protein-coupled receptor signaling.Mol Endocrinol. 2015 Jun;29(6):814-30. doi: 10.1210/me.2015-1091. Epub 2015 May 5. Mol Endocrinol. 2015. PMID: 25942107 Free PMC article. Review.
-
Nanobody-based RFP-dependent Cre recombinase for selective anterograde tracing in RFP-expressing transgenic animals.Commun Biol. 2022 Sep 16;5(1):979. doi: 10.1038/s42003-022-03944-2. Commun Biol. 2022. PMID: 36114373 Free PMC article.
-
Cardiac cAMP-PKA Signaling Compartmentalization in Myocardial Infarction.Cells. 2021 Apr 16;10(4):922. doi: 10.3390/cells10040922. Cells. 2021. PMID: 33923648 Free PMC article. Review.
References
-
- Lohse MJ, Nuber S, Hoffmann C. Fluorescence/bioluminescence resonance energy transfer techniques to study G-protein-coupled receptor activation and signaling. Pharmacol. Rev. 2012;64:299–336. - PubMed
-
- Shenoy SK, Lefkowitz RJ. Seven-transmembrane receptor signaling through β-arrestin. Sci. STKE 2005. 2005:cm10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 DA010154/DA/NIDA NIH HHS/United States
- R37 DA010711/DA/NIDA NIH HHS/United States
- T32 GM007767/GM/NIGMS NIH HHS/United States
- R01 GM083118/GM/NIGMS NIH HHS/United States
- R29 DA010711/DA/NIDA NIH HHS/United States
- DA010711/DA/NIDA NIH HHS/United States
- P01 NS053709/NS/NINDS NIH HHS/United States
- R01 DA012864/DA/NIDA NIH HHS/United States
- R01 DA010711/DA/NIDA NIH HHS/United States
- R01 GM068603/GM/NIGMS NIH HHS/United States
- F32 DA029993/DA/NIDA NIH HHS/United States
- GM083118/GM/NIGMS NIH HHS/United States
- DA012864/DA/NIDA NIH HHS/United States

