TRPM2 links oxidative stress to NLRP3 inflammasome activation
- PMID: 23511475
- PMCID: PMC3605705
- DOI: 10.1038/ncomms2608
TRPM2 links oxidative stress to NLRP3 inflammasome activation
Abstract
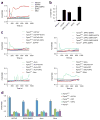
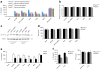
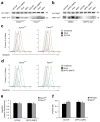
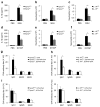
Exposure to particulate crystals can induce oxidative stress in phagocytes, which triggers NLRP3 inflammasome-mediated interleukin-1β secretion to initiate undesirable inflammatory responses that are associated with both autoinflammatory and metabolic diseases. Although mitochondrial reactive oxygen species have a central role in NLRP3 inflammasome activation, how reactive oxygen species signal assembly of the NLRP3 inflammasome remains elusive. Here, we identify liposomes as novel activators of the NLRP3 inflammasome and further demonstrate that liposome-induced inflammasome activation also requires mitochondrial reactive oxygen species. Moreover, we find that stimulation with liposomes/crystals induced reactive oxygen species-dependent calcium influx via the TRPM2 channel and that macrophages deficient in TRPM2 display drastically impaired NLRP3 inflammasome activation and interleukin-1β secretion. Consistently, Trpm2(-/-) mice are resistant to crystal-/liposome-induced interleukin-1β-mediated peritonitis in vivo. Together, these results identify TRPM2 as a key factor that links oxidative stress to the NLRP3 inflammasome activation. Therefore, targeting TRPM2 may be effective for the treatment of NLRP3 inflammasome-associated inflammatory disorders.
Conflict of interest statement
Competing financial interests: The authors declare no competing financial interests.
Figures


Similar articles
-
TRPM2 regulates TXNIP-mediated NLRP3 inflammasome activation via interaction with p47 phox under high glucose in human monocytic cells.Sci Rep. 2016 Oct 12;6:35016. doi: 10.1038/srep35016. Sci Rep. 2016. PMID: 27731349 Free PMC article.
-
Hyperosmotic Stress-Induced TRPM2 Channel Activation Stimulates NLRP3 Inflammasome Activity in Primary Human Corneal Epithelial Cells.Invest Ophthalmol Vis Sci. 2018 Jul 2;59(8):3259-3268. doi: 10.1167/iovs.18-23965. Invest Ophthalmol Vis Sci. 2018. PMID: 29971445
-
Reactive oxygen species activated NLRP3 inflammasomes prime environment-induced murine dry eye.Exp Eye Res. 2014 Aug;125:1-8. doi: 10.1016/j.exer.2014.05.001. Epub 2014 May 14. Exp Eye Res. 2014. PMID: 24836981
-
TRPM2, linking oxidative stress and Ca2+ permeation to NLRP3 inflammasome activation.Curr Opin Immunol. 2020 Feb;62:131-135. doi: 10.1016/j.coi.2020.01.005. Epub 2020 Feb 12. Curr Opin Immunol. 2020. PMID: 32058297 Free PMC article. Review.
-
Stressing out the mitochondria: Mechanistic insights into NLRP3 inflammasome activation.J Leukoc Biol. 2019 Feb;105(2):377-399. doi: 10.1002/JLB.MR0318-124R. Epub 2018 Dec 27. J Leukoc Biol. 2019. PMID: 30589456 Review.
Cited by
-
2'-deoxy-ADPR activates human TRPM2 faster than ADPR and thereby induces higher currents at physiological Ca2+ concentrations.Front Immunol. 2024 Jan 22;15:1294357. doi: 10.3389/fimmu.2024.1294357. eCollection 2024. Front Immunol. 2024. PMID: 38318185 Free PMC article.
-
Redox-sensitive transient receptor potential channels in oxygen sensing and adaptation.Pflugers Arch. 2016 Jan;468(1):85-97. doi: 10.1007/s00424-015-1716-2. Epub 2015 Jul 7. Pflugers Arch. 2016. PMID: 26149285 Free PMC article. Review.
-
Activation and regulation of the inflammasomes.Nat Rev Immunol. 2013 Jun;13(6):397-411. doi: 10.1038/nri3452. Nat Rev Immunol. 2013. PMID: 23702978 Free PMC article. Review.
-
DAMP-sensing receptors in sterile inflammation and inflammatory diseases.Nat Rev Immunol. 2020 Feb;20(2):95-112. doi: 10.1038/s41577-019-0215-7. Epub 2019 Sep 26. Nat Rev Immunol. 2020. PMID: 31558839 Review.
-
NLRP3 inflammasome and host protection against bacterial infection.J Korean Med Sci. 2013 Oct;28(10):1415-23. doi: 10.3346/jkms.2013.28.10.1415. Epub 2013 Sep 25. J Korean Med Sci. 2013. PMID: 24133343 Free PMC article. Review.
References
-
- Allison AG, Gregoriadis G. Liposomes as immunological adjuvants. Nature. 1974;252:252. - PubMed
-
- Al-Jamal WT, Kostarelos K. Liposomes: from a clinically established drug delivery system to a nanoparticle platform for theranostic nanomedicine. Acc Chem Res. 2011;44:1094–104. - PubMed
-
- Slingerland M, Guchelaar HJ, Gelderblom H. Liposomal drug formulations in cancer therapy: 15 years along the road. Drug Discov Today. 2012;17:160–6. - PubMed
-
- Christensen D, et al. Cationic liposomes as vaccine adjuvants. Expert Rev Vaccines. 2007;6:785–96. - PubMed
-
- Yan W, Chen W, Huang L. Mechanism of adjuvant activity of cationic liposome: phosphorylation of a MAP kinase, ERK and induction of chemokines. Mol Immunol. 2007;44:3672–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

