Dephosphorylation of the RNA sensors RIG-I and MDA5 by the phosphatase PP1 is essential for innate immune signaling
- PMID: 23499489
- PMCID: PMC3616631
- DOI: 10.1016/j.immuni.2012.11.018
Dephosphorylation of the RNA sensors RIG-I and MDA5 by the phosphatase PP1 is essential for innate immune signaling
Abstract
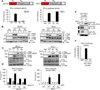
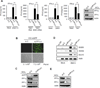
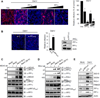
RIG-I and MDA5 have emerged as key cytosolic sensors for the detection of RNA viruses and lead to antiviral interferon (IFN) production. Recent studies have highlighted the importance of posttranslational modifications for controlling RIG-I antiviral activity. However, the regulation of MDA5 signal-transducing ability remains unclear. Here, we show that MDA5 signaling activity is regulated by a dynamic balance between phosphorylation and dephosphorylation of its caspase recruitment domains (CARDs). Employing a phosphatome RNAi screen, we identified PP1α and PP1γ as the primary phosphatases that are responsible for MDA5 and RIG-I dephosphorylation and that lead to their activation. Silencing of PP1α and PP1γ enhanced RIG-I and MDA5 CARD phosphorylation and reduced antiviral IFN-β production. PP1α- and PP1γ-depleted cells were impaired in their ability to induce IFN-stimulated gene expression, which resulted in enhanced RNA virus replication. This work identifies PP1α and PP1γ as regulators of antiviral innate immune responses to various RNA viruses, including influenza virus, paramyxovirus, dengue virus, and picornavirus.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures




Comment in
-
Phosphorylation and dephosphorylation of the RIG-I-like receptors: a safety latch on a fateful pathway.Immunity. 2013 Mar 21;38(3):402-3. doi: 10.1016/j.immuni.2013.02.014. Immunity. 2013. PMID: 23521878
Similar articles
-
LGP2 synergy with MDA5 in RLR-mediated RNA recognition and antiviral signaling.Cytokine. 2015 Aug;74(2):198-206. doi: 10.1016/j.cyto.2015.02.010. Epub 2015 Mar 18. Cytokine. 2015. PMID: 25794939 Free PMC article. Review.
-
GEF-H1 controls microtubule-dependent sensing of nucleic acids for antiviral host defenses.Nat Immunol. 2014 Jan;15(1):63-71. doi: 10.1038/ni.2766. Epub 2013 Nov 24. Nat Immunol. 2014. PMID: 24270516 Free PMC article.
-
LGP2 is a positive regulator of RIG-I- and MDA5-mediated antiviral responses.Proc Natl Acad Sci U S A. 2010 Jan 26;107(4):1512-7. doi: 10.1073/pnas.0912986107. Epub 2010 Jan 8. Proc Natl Acad Sci U S A. 2010. PMID: 20080593 Free PMC article.
-
RIG-I/MDA5/MAVS are required to signal a protective IFN response in rotavirus-infected intestinal epithelium.J Immunol. 2011 Feb 1;186(3):1618-26. doi: 10.4049/jimmunol.1002862. Epub 2010 Dec 27. J Immunol. 2011. PMID: 21187438
-
Sensing of viral nucleic acids by RIG-I: from translocation to translation.Eur J Cell Biol. 2012 Jan;91(1):78-85. doi: 10.1016/j.ejcb.2011.01.015. Epub 2011 Apr 14. Eur J Cell Biol. 2012. PMID: 21496944 Free PMC article. Review.
Cited by
-
SARS-CoV-2 Nucleocapsid Protein Antagonizes GADD34-Mediated Innate Immune Pathway through Atypical Foci.Molecules. 2024 Oct 10;29(20):4792. doi: 10.3390/molecules29204792. Molecules. 2024. PMID: 39459161 Free PMC article.
-
IFIH1 (MDA5) is required for innate immune detection of intron-containing RNA expressed from the HIV-1 provirus.bioRxiv [Preprint]. 2023 Dec 12:2023.11.17.567619. doi: 10.1101/2023.11.17.567619. bioRxiv. 2023. Update in: Proc Natl Acad Sci U S A. 2024 Jul 16;121(29):e2404349121. doi: 10.1073/pnas.2404349121. PMID: 38014177 Free PMC article. Updated. Preprint.
-
Transcriptome markers of viral persistence in naturally-infected andes virus (bunyaviridae) seropositive long-tailed pygmy rice rats.PLoS One. 2015 Apr 9;10(4):e0122935. doi: 10.1371/journal.pone.0122935. eCollection 2015. PLoS One. 2015. PMID: 25856432 Free PMC article.
-
Direct Interaction of Coronavirus Nonstructural Protein 3 with Melanoma Differentiation-Associated Gene 5 Modulates Type I Interferon Response during Coronavirus Infection.Int J Mol Sci. 2022 Oct 2;23(19):11692. doi: 10.3390/ijms231911692. Int J Mol Sci. 2022. PMID: 36232993 Free PMC article.
-
LGP2 synergy with MDA5 in RLR-mediated RNA recognition and antiviral signaling.Cytokine. 2015 Aug;74(2):198-206. doi: 10.1016/j.cyto.2015.02.010. Epub 2015 Mar 18. Cytokine. 2015. PMID: 25794939 Free PMC article. Review.
References
-
- Aebersold R, Goodlett DR. Mass spectrometry in proteomics. Chem Rev. 2001;101:269–295. - PubMed
-
- Barbalat R, Ewald SE, Mouchess ML, Barton GM. Nucleic acid recognition by the innate immune system. Annu Rev Immunol. 2011;29:185–214. - PubMed
-
- Cohen PT. Protein phosphatase 1--targeted in many directions. J Cell Sci. 2002;115:241–256. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

