The US27 gene product of human cytomegalovirus enhances signaling of host chemokine receptor CXCR4
- PMID: 23490053
- PMCID: PMC3639318
- DOI: 10.1016/j.virol.2013.02.006
The US27 gene product of human cytomegalovirus enhances signaling of host chemokine receptor CXCR4
Abstract
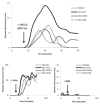
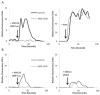
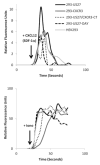
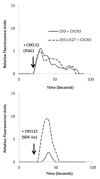
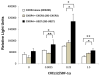
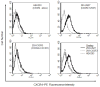
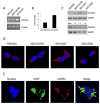
Human cytomegalovirus (HCMV) is a member of the Herpesviridae family that manipulates host immune responses and establishes life-long latent infection, in part through mimicry of cytokines, chemokines, and chemokine receptors. The HCMV US27 gene product is a putative chemokine receptor with no known ligands. We generated a stable US27 cell line to screen for chemokine ligands but unexpectedly found that US27 potentiated the activity of an endogenous human chemokine receptor, CXCR4. Cells expressing both US27 and CXCR4 exhibited greater calcium mobilization and enhanced chemotaxis in response to CXCL12/SDF-1α than controls. Quantitative RT-PCR revealed a significant increase in CXCR4 expression when US27 was present, and elevated CXCR4 receptor levels were detected via flow cytometry, western blot, and immunofluorescence microscopy. Potentiation of CXCR4 signaling by US27 could represent a novel strategy by which HCMV targets virus-infected cells to the bone marrow in order to expand the reservoir of latently infected cells.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
The human cytomegalovirus chemokine receptor homolog encoded by US27.Virus Genes. 2017 Aug;53(4):516-521. doi: 10.1007/s11262-017-1462-y. Epub 2017 Apr 26. Virus Genes. 2017. PMID: 28447191 Review.
-
Human Cytomegalovirus UL111A and US27 Gene Products Enhance the CXCL12/CXCR4 Signaling Axis via Distinct Mechanisms.J Virol. 2018 Feb 12;92(5):e01981-17. doi: 10.1128/JVI.01981-17. Print 2018 Mar 1. J Virol. 2018. PMID: 29237840 Free PMC article.
-
The Human Cytomegalovirus US27 Gene Product Constitutively Activates Antioxidant Response Element-Mediated Transcription through Gβγ, Phosphoinositide 3-Kinase, and Nuclear Respiratory Factor 1.J Virol. 2018 Nov 12;92(23):e00644-18. doi: 10.1128/JVI.00644-18. Print 2018 Dec 1. J Virol. 2018. PMID: 30209167 Free PMC article.
-
Effect of human cytomegalovirus (HCMV) US27 on CXCR4 receptor internalization measured by fluorogen-activating protein (FAP) biosensors.PLoS One. 2017 Feb 16;12(2):e0172042. doi: 10.1371/journal.pone.0172042. eCollection 2017. PLoS One. 2017. PMID: 28207860 Free PMC article.
-
Viral chemokine receptors and chemokines in human cytomegalovirus trafficking and interaction with the immune system. CMV chemokine receptors.Curr Top Microbiol Immunol. 2002;269:203-34. doi: 10.1007/978-3-642-59421-2_13. Curr Top Microbiol Immunol. 2002. PMID: 12224510 Review.
Cited by
-
Functional annotation of human cytomegalovirus gene products: an update.Front Microbiol. 2014 May 19;5:218. doi: 10.3389/fmicb.2014.00218. eCollection 2014. Front Microbiol. 2014. PMID: 24904534 Free PMC article. Review.
-
Evolution of the ability to modulate host chemokine networks via gene duplication in human cytomegalovirus (HCMV).Infect Genet Evol. 2017 Jul;51:46-53. doi: 10.1016/j.meegid.2017.03.013. Epub 2017 Mar 14. Infect Genet Evol. 2017. PMID: 28315475 Free PMC article.
-
Modulation of cellular signaling by herpesvirus-encoded G protein-coupled receptors.Front Pharmacol. 2015 Mar 9;6:40. doi: 10.3389/fphar.2015.00040. eCollection 2015. Front Pharmacol. 2015. PMID: 25805993 Free PMC article. Review.
-
The human cytomegalovirus chemokine receptor homolog encoded by US27.Virus Genes. 2017 Aug;53(4):516-521. doi: 10.1007/s11262-017-1462-y. Epub 2017 Apr 26. Virus Genes. 2017. PMID: 28447191 Review.
-
Development of a replication-competent lentivirus assay for dendritic cell-targeting lentiviral vectors.Mol Ther Methods Clin Dev. 2015 May 13;2:15017. doi: 10.1038/mtm.2015.17. eCollection 2015. Mol Ther Methods Clin Dev. 2015. PMID: 26029728 Free PMC article.
References
-
- Babcock GJ, Farzan M, Sodroski J. Ligand-independent dimerization of CXCR4, a principal HIV-1 coreceptor. J Biol Chem. 2003;278:3378–3385. - PubMed
-
- Beck S, Barrell BG. Human cytomegalovirus encodes a glycoprotein homologous to MHC class-I antigens. Nature. 1988;331:269–272. - PubMed
-
- Bodaghi B, Jones TR, Zipeto D, Vita C, Sun L, Laurent L, Arenzana-Seisdedos F, Virelizier JL, Michelson S. Chemokine sequestration by viral chemoreceptors as a novel viral escape strategy: withdrawal of chemokines from the environment of cytomegalovirus infected cells. J Exp Med. 1998;188:855–866. - PMC - PubMed
-
- Burns JM, Lewis GK. Improved measurement of calcium mobilization by flow cytometry. Biotechniques. 1997;23:1022–1024. 1026. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

