Proteolysis of BB0323 results in two polypeptides that impact physiologic and infectious phenotypes in Borrelia burgdorferi
- PMID: 23489252
- PMCID: PMC3633617
- DOI: 10.1111/mmi.12202
Proteolysis of BB0323 results in two polypeptides that impact physiologic and infectious phenotypes in Borrelia burgdorferi
Abstract
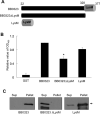
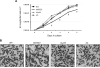
Borrelia burgdorferi gene product BB0323 is required for cell fission and pathogen persistence in vivo. Here, we show that BB0323, which is conserved among globally prevalent infectious strains, supports normal spirochaete growth and morphology even at early phases of cell division. We demonstrate that native BB0323 undergoes proteolytic processing at the C-terminus, at a site after the first 202 N-terminal amino acids. We further identified a periplasmic BB0323 binding protein in B. burgdorferi, annotated as BB0104, having serine protease activity responsible for the primary cleavage of BB0323 to produce discrete N- and C-terminal polypeptides. These two BB0323 polypeptides interact with each other, and either individually or as a complex, are associated with multiple functions in spirochaete biology and infectivity. While N-terminal BB0323 is adequate to support cell fission, the C-terminal LysM domain is dispensable for this process, despite its ability to bind B. burgdorferi peptidoglycan. However, the LysM domain or the precisely processed BB0323 product is essential for mammalian infection. As BB0323 is a membrane protein crucial for B. burgdorferi survival in vivo, exploring its function may suggest novel ways to interrupt infection while enhancing our understanding of the intricate spirochaete fission process.
© 2013 Blackwell Publishing Ltd.
Figures



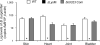
Similar articles
-
Fluorescent Proteins, Promoters, and Selectable Markers for Applications in the Lyme Disease Spirochete Borrelia burgdorferi.Appl Environ Microbiol. 2018 Nov 30;84(24):e01824-18. doi: 10.1128/AEM.01824-18. Print 2018 Dec 15. Appl Environ Microbiol. 2018. PMID: 30315081 Free PMC article.
-
BB0323 and novel virulence determinant BB0238: Borrelia burgdorferi proteins that interact with and stabilize each other and are critical for infectivity.J Infect Dis. 2015 Feb 1;211(3):462-71. doi: 10.1093/infdis/jiu460. Epub 2014 Aug 19. J Infect Dis. 2015. PMID: 25139020 Free PMC article.
-
BB0323 function is essential for Borrelia burgdorferi virulence and persistence through tick-rodent transmission cycle.J Infect Dis. 2009 Oct 15;200(8):1318-30. doi: 10.1086/605846. J Infect Dis. 2009. PMID: 19754308 Free PMC article.
-
Crystal structure of the N-terminal domain of the major virulence factor BB0323 from the Lyme disease agent Borrelia burgdorferi.Acta Crystallogr D Struct Biol. 2019 Sep 1;75(Pt 9):825-830. doi: 10.1107/S2059798319010751. Epub 2019 Aug 22. Acta Crystallogr D Struct Biol. 2019. PMID: 31478905
-
Controlled Proteolysis of an Essential Virulence Determinant Dictates Infectivity of Lyme Disease Pathogens.Infect Immun. 2022 May 19;90(5):e0005922. doi: 10.1128/iai.00059-22. Epub 2022 Apr 13. Infect Immun. 2022. PMID: 35416705 Free PMC article.
Cited by
-
Fluorescent Proteins, Promoters, and Selectable Markers for Applications in the Lyme Disease Spirochete Borrelia burgdorferi.Appl Environ Microbiol. 2018 Nov 30;84(24):e01824-18. doi: 10.1128/AEM.01824-18. Print 2018 Dec 15. Appl Environ Microbiol. 2018. PMID: 30315081 Free PMC article.
-
A chitin deacetylase-like protein is a predominant constituent of tick peritrophic membrane that influences the persistence of Lyme disease pathogens within the vector.PLoS One. 2013 Oct 17;8(10):e78376. doi: 10.1371/journal.pone.0078376. eCollection 2013. PLoS One. 2013. PMID: 24147133 Free PMC article.
-
HtrA of Borrelia burgdorferi Leads to Decreased Swarm Motility and Decreased Production of Pyruvate.mBio. 2018 Jul 10;9(4):e01136-18. doi: 10.1128/mBio.01136-18. mBio. 2018. PMID: 29991588 Free PMC article.
-
Application of temperature-responsive HIS-tag fluorophores to differential scanning fluorimetry screening of small molecule libraries.Front Pharmacol. 2022 Nov 24;13:1040039. doi: 10.3389/fphar.2022.1040039. eCollection 2022. Front Pharmacol. 2022. PMID: 36506591 Free PMC article.
-
Membrane directed expression in Escherichia coli of BBA57 and other virulence factors from the Lyme disease agent Borrelia burgdorferi.Sci Rep. 2019 Nov 26;9(1):17606. doi: 10.1038/s41598-019-53830-x. Sci Rep. 2019. PMID: 31772280 Free PMC article.
References
-
- Beck G, Benach JL, Habicht GS. Isolation, preliminary chemical characterization, and biological activity of Borrelia burgdorferi peptidoglycan. Biochem Biophys Res Commun. 1990;167:89–95. - PubMed
-
- Bergström S, Zückert WR. Structure, Function and Biogenesis of the Borrelia Cell Envelope. In: Samuels DS, Radolf JD, editors. Borrelia, Molecular Biology, Host Interaction and Pathogenesis. Caister Academic Press; Norfolk, UK: 2010. pp. 139–166.
-
- Buist G, Steen A, Kok J, Kuipers OP. LysM, a widely distributed protein motif for binding to (peptido)glycans. Mol Microbiol. 2008;68:838–847. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

