Phase I study of the CD40 agonist antibody CP-870,893 combined with carboplatin and paclitaxel in patients with advanced solid tumors
- PMID: 23483678
- PMCID: PMC3583942
- DOI: 10.4161/onci.23033
Phase I study of the CD40 agonist antibody CP-870,893 combined with carboplatin and paclitaxel in patients with advanced solid tumors
Abstract
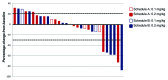
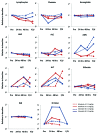
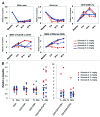
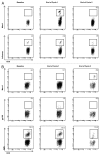
CD40 is a cell-surface molecule that critically regulates immune responses. CP-870,893 is a fully human, CD40-specific agonist monoclonal antibody (mAb) exerting clinical antineoplastic activity. Here, the safety of CP-870,893 combined with carboplatin and paclitaxel was assessed in a Phase I study. Patients with advanced solid tumors received standard doses of paclitaxel and carboplatin on day 1 followed by either 0.1 mg/Kg or 0.2 mg/Kg CP-870,893 on day 3 (Schedule A) or day 8 (Schedule B), repeated every 21 d. The primary objective was to determine safety and maximum-tolerated dose (MTD) of CP-870,893. Secondary objectives included the evaluation of antitumor responses, pharmacokinetics and immune modulation. Thirty-two patients were treated with CP-870,893, 16 patients on each schedule. Two dose-limiting toxicities were observed (grade 3 cytokine release and transient ischemic attack), each at the 0.2 mg/Kg dose level, which was estimated to be the MTD. The most common treatment-related adverse event was fatigue (81%). Of 30 evaluable patients, 6 (20%) exhibited partial responses constituting best responses as defined by RECIST. Following CP-870,893 infusion, the peripheral blood manifested an acute depletion of B cells associated with upregulation of immune co-stimulatory molecules. T-cell numbers did not change significantly from baseline, but transient tumor-specific T-cell responses were observed in a small number of evaluable patients. The CD40 agonist mAb CP-870,893, given on either of two schedules in combination with paclitaxel and carboplatin, was safe for patients affected with advanced solid tumors. Biological and clinical responses were observed, providing a rationale for Phase II studies.
Trial registration: ClinicalTrials.gov NCT00607048.
Keywords: CD40; CP-870,893; T cells; chemotherapy; clinical trial; monoclonal antibody.
Figures
Similar articles
-
Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody.J Clin Oncol. 2007 Mar 1;25(7):876-83. doi: 10.1200/JCO.2006.08.3311. J Clin Oncol. 2007. PMID: 17327609 Clinical Trial.
-
Phase I study of oral ridaforolimus in combination with paclitaxel and carboplatin in patients with solid tumor cancers.BMC Cancer. 2017 Jun 8;17(1):407. doi: 10.1186/s12885-017-3394-2. BMC Cancer. 2017. PMID: 28595616 Free PMC article. Clinical Trial.
-
Sunitinib in combination with paclitaxel plus carboplatin in patients with advanced solid tumors: phase I study results.Cancer Chemother Pharmacol. 2011 Sep;68(3):703-12. doi: 10.1007/s00280-010-1536-1. Epub 2010 Dec 8. Cancer Chemother Pharmacol. 2011. PMID: 21140147 Free PMC article. Clinical Trial.
-
Phase I and pharmacokinetic trial of carboplatin and albumin-bound paclitaxel, ABI-007 (Abraxane) on three treatment schedules in patients with solid tumors.Cancer Chemother Pharmacol. 2007 Oct;60(5):759-66. doi: 10.1007/s00280-007-0423-x. Epub 2007 Feb 7. Cancer Chemother Pharmacol. 2007. PMID: 17285317 Free PMC article. Clinical Trial.
-
Phase I/II study of the combination of carboplatin and paclitaxel as first-line chemotherapy in patients with advanced epithelial ovarian cancer.Ann Oncol. 1997 Apr;8(4):355-61. doi: 10.1023/a:1008267419453. Ann Oncol. 1997. PMID: 9209665 Clinical Trial.
Cited by
-
Prognostic significance and targeting tumor-associated macrophages in cancer: new insights and future perspectives.Breast Cancer. 2021 May;28(3):539-555. doi: 10.1007/s12282-021-01231-2. Epub 2021 Mar 4. Breast Cancer. 2021. PMID: 33661479 Review.
-
Trial Watch-Oncolytic viruses and cancer therapy.Oncoimmunology. 2015 Dec 8;5(2):e1117740. doi: 10.1080/2162402X.2015.1117740. eCollection 2016 Feb. Oncoimmunology. 2015. PMID: 27057469 Free PMC article. Review.
-
Neoadjuvant Selicrelumab, an Agonist CD40 Antibody, Induces Changes in the Tumor Microenvironment in Patients with Resectable Pancreatic Cancer.Clin Cancer Res. 2021 Aug 15;27(16):4574-4586. doi: 10.1158/1078-0432.CCR-21-1047. Epub 2021 Jun 10. Clin Cancer Res. 2021. PMID: 34112709 Free PMC article. Clinical Trial.
-
Immunotherapies Targeting Tumor-Associated Macrophages (TAMs) in Cancer.Pharmaceutics. 2024 Jun 27;16(7):865. doi: 10.3390/pharmaceutics16070865. Pharmaceutics. 2024. PMID: 39065562 Free PMC article. Review.
-
Programming CAR T cells to enhance anti-tumor efficacy through remodeling of the immune system.Front Med. 2020 Dec;14(6):726-745. doi: 10.1007/s11684-020-0746-0. Epub 2020 Aug 13. Front Med. 2020. PMID: 32794014 Review.
References
-
- van Kooten C, Banchereau J. CD40-CD40 ligand. J Leukoc Biol. 2000;67:2–17. - PubMed
Publication types
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
